Catabolism
Catabolism (/kəˈtæbəlɪsm/) is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions.[1] Catabolism breaks down large molecules (such as polysaccharides, lipids, nucleic acids and proteins) into smaller units (such as monosaccharides, fatty acids, nucleotides, and amino acids, respectively). Catabolism is the breaking-down aspect of metabolism, whereas anabolism is the building-up aspect.
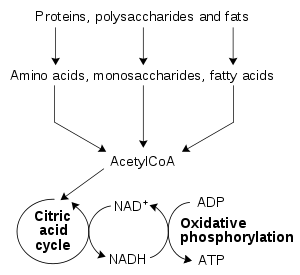
Cells use the monomers released from breaking down polymers to either construct new polymer molecules, or degrade the monomers further to simple waste products, releasing energy. Cellular wastes include lactic acid, acetic acid, carbon dioxide, ammonia, and urea. The creation of these wastes is usually an oxidation process involving a release of chemical free energy, some of which is lost as heat, but the rest of which is used to drive the synthesis of adenosine triphosphate (ATP). This molecule acts as a way for the cell to transfer the energy released by catabolism to the energy-requiring reactions that make up anabolism. (Catabolism is seen as destructive metabolism and anabolism as constructive metabolism). Catabolism therefore provides the chemical energy necessary for the maintenance and growth of cells. Examples of catabolic processes include glycolysis, the citric acid cycle, the breakdown of muscle protein in order to use amino acids as substrates for gluconeogenesis, the breakdown of fat in adipose tissue to fatty acids, and oxidative deamination of neurotransmitters by monoamine oxidase.
Etymology
The word catabolism is from New Latin, which got the roots from Greek: κάτω kato, "downward" and βάλλειν ballein, "to throw".
See also
- Autophagy
- Dehydration synthesis
- Hydrolysis
- Nocturnal post absorptive catabolism
- Psilacetin § Pharmacology
- Sarcopenia
References
- de Bolster, M.W.G. (1997). "Glossary of Terms Used in Bioinorganic Chemistry: Catabolism". International Union of Pure and Applied Chemistry. Archived from the original on 2017-01-21. Retrieved 2007-10-30.
External links
