Budesonide
Budesonide (BUD), sold under the brand name Pulmicort among others, is a medication of the corticosteroid type.[1] It is available as an inhaler, pill, nasal spray, and rectal forms.[1][2] The inhaled form is used in the long-term management of asthma and chronic obstructive pulmonary disease (COPD).[1][3][4] The nasal spray is used for allergic rhinitis and nasal polyps.[2][5] The pills in a delayed release form and rectal forms may be used for inflammatory bowel disease including Crohn's disease, ulcerative colitis and microscopic colitis.[6][7][8]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Pulmicort, Rhinocort, Entocort, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608007 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, nasal, tracheal, rectal, inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 10-20% (first pass effect) |
| Protein binding | 85-90% |
| Metabolism | Hepatic CYP3A4 |
| Elimination half-life | 2.0-3.6 hours |
| Excretion | Urine, feces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.927 |
| Chemical and physical data | |
| Formula | C25H34O6 |
| Molar mass | 430.534 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
InChI
| |
| | |
Common side effects with the inhaled form include respiratory infections, cough, and headaches.[1] Common side effects with the pills include feeling tired, vomiting, and joint pains.[1] Serious side effects include an increased risk of infection, loss of bone strength, and cataracts.[1] Long-term use of the pill form may cause adrenal insufficiency.[1] Stopping the pills suddenly following long-term use may therefore be dangerous.[1] The inhaled form is generally safe in pregnancy.[1] Budesonide is mainly acting as a glucocorticoid.[1]
Budesonide was initially patented in 1973.[9] Commercial use as an asthma medication began in 1981.[10] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[11] Some forms are available as a generic medication.[12] The wholesale price in the developing world for an inhaler containing 200 doses is about US$5 to US$7 as of 2014.[13] As of 2015, the cost for a typical month of the inhaler medication in the United States is US$100 to US$200.[12] In 2017, it was the 190th most commonly prescribed medication in the United States, with more than three million prescriptions.[14][15]
Medical uses
Asthma
Budesonide is nebulized for maintenance and prophylactic treatment of asthma including patients who require oral corticosteroids and those who may benefit from a systemic dose reduction.[16]
Inflammatory bowel disease
Formulations of delayed-release budesonide are an effective treatment for mild-to-moderately active Crohn's disease involving the ileum and/or ascending colon.[17] A Cochrane review found evidence for up to three months (but not longer) of maintenance of remission in Crohn's disease.[18]
Budesonide assists in the induction of remission in people with active ulcerative colitis.[19]
Budesonide is highly effective and recommended as the drug of choice in microscopic colitis, for induction and maintenance of remission, and for both the lymphocytic colitis and collagenous colitis forms.[20]
Allergic rhinitis
Budesonide in the form of nasal sprays is a treatment for allergic rhinitis.[21]
Side effects
Budesonide may cause:[24]
- Nose irritation or burning
- Bleeding or sores in the nose
- Lightheadedness
- Upset stomach
- Cough
- Hoarseness
- Dry mouth
- Rash
- Sore throat
- Bad taste in mouth
- Change in mucus
- Blurred vision [25]
In addition, the following symptoms should be reported immediately:
- Difficulty breathing or swelling of the face
- White patches in the throat, mouth, or nose
- Irregular menstrual periods
- Severe acne
- On rare occasions, behavioral changes (mostly affecting children)[24]
Contraindications
Budesonide is contraindicated as a primary treatment of status asthmaticus or other acute episode of asthma where intensive measures are required.[26] It is also contraindicated for patients who have hypersensitivity to budesonide.[27]
Interactions
Those taking tablets or capsules orally should avoid grapefruit juice and echinacea.
- Grapefruit juice may double bioavailability of oral budesonide.
- Echinacea diminishes bioavailability.
Also, high-fat meals delay absorption but do not impede absorption.
Pharmacology
Budesonide is an agonist of glucocorticoid receptors. Among its effects are:
- Controls the rate of protein synthesis.
- Depresses the migration of polymorphonuclear leukocytes and fibroblasts.
- Reverses capillary permeability and lysosomal stabilization at the cellular level to prevent or control inflammation.
- Has a potent glucocorticoid activity and weak mineralocorticoid activity.
Pharmacokinetics
- Onset of action: Nebulization: 2–8 days; Inhalation: 24 hours; Nasal: 10 hours
- Peak effect: Nebulization: 4–6 weeks; Inhalation: 1–2 weeks
- Distribution: 2.2-3.9 L/kg
- Protein binding: 85% to 90%
- Metabolism: Hepatic via CYP3A4 to two metabolites: 16 alpha-hydroxyprednisolone and 6 beta-hydroxybudesonide; minor activity
- Bioavailability: Limited by high first-pass effect; Capsule: 9% to 21%; Nebulization: 6%; Inhalation: 6% to 13%
- Half-life elimination: 2–3.6 hours
- Time to peak: Capsule: 0.5–10 hours (variable in Crohn's disease); Nebulization: 10–30 minutes; Inhalation: 1–2 hours; Tablet: 7.4-19.2 hours
- Excretion: urine (60%) and feces as metabolites.
Chemistry
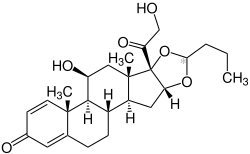
Budesonide, also known as 11β,21-dihydroxy-16α,17α-(butylidenebis(oxy))pregna-1,4-diene-3,20-dione, is a synthetic pregnane steroid and non-halogenated cyclic ketal corticosteroid.[28][29] It is the C16α hydroxyl, C16α,17α cyclic ketal with butyraldehyde derivative of prednisolone (11β,17α,21-trihydroxypregna-1,4-diene-3,20-dione).[28][29]
Stereoisomerism
| Budesonide (2 stereoisomers) | |
|---|---|
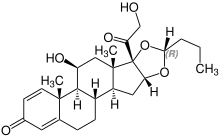 (22R)-configuration |
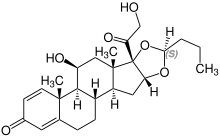 (22S)-configuration |
Society and culture
Brand names

Aeronide (TH); Aquacort (DE); B Cort (CO); Bronex (PH); Budair (MY); Budecort DP (MY); Budenofalk (DE, GB, HK, KP, PH, SG); Budeson (AR); Budeson Aqua (AR); BudeSpray (TH); Budiair (KP); Budicort Respules (IL); Budinide (KSA); Bunase (TH); Clebudan (CN); Cortiment (GB); Cycortide (HK); Denecort (PH); Duasma (TW); Eltair (MY); Entocort (AR, AT, BE, BR, CH, CZ, DK, FI, FR, GB, HK, IE, IL, IT, KP, NL, NO, PL, PT, SE, TR); Giona Easyhaler (MY, SG, TH); Inflammide (PE); Miflonid (CZ); Miflonide (BE, DE, IL, IT, NZ, PT); Neumocort (PY); Novopulmon (DE, FR); Pulmicon Susp for Nebulizer (KP); Pulmicort (AT, BE, BG, BR, CH, CL, CN, CO, CR, CZ, DE, DK, DO, EE, FI, FR, GB, GR, GT, HN, ID, IN, NI, NL, NO, PA, PK, PL, PT, RU, SE, SV, TR, TW, UY, VE, ZA); Pulmicort Nasal Turbohaler (CL, KE, MU, NG); Pulmicort Turbuhaler (KE, MU, NG); Rafton (FR); Rhinocort (AU); Rhinocort Aqua (HK); Rhinoside (GR); Symbicort (FR, US, ZA) Uceris (US)
See also
- Azelastine
- Fluticasone propionate
References
- "Budesonide". The American Society of Health-System Pharmacists. Archived from the original on 28 November 2015. Retrieved 2 December 2015.
- "Budesonide eent". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
- De Coster, DA; Jones, M (2014). "Tailoring of corticosteroids in COPD management". Current Respiratory Care Reports. 3 (3): 121–132. doi:10.1007/s13665-014-0084-2. PMC 4113685. PMID 25089228.
- Christophi, GP; Rengarajan, A; Ciorba, MA (2016). "Rectal budesonide and mesalamine formulations in active ulcerative proctosigmoiditis: efficacy, tolerance, and treatment approach". Clinical and Experimental Gastroenterology. 9: 125–30. doi:10.2147/CEG.S80237. PMC 4876845. PMID 27274301.
- Rudmik, L; Schlosser, RJ; Smith, TL; Soler, ZM (July 2012). "Impact of topical nasal steroid therapy on symptoms of nasal polyposis: a meta-analysis". The Laryngoscope. 122 (7): 1431–7. doi:10.1002/lary.23259. PMID 22410935.
- Silverman J, Otley A (2011). "Budesonide in the treatment of inflammatory bowel disease". Expert Rev Clin Immunol. 7 (4): 419–28. doi:10.1586/eci.11.34. PMID 21790284.
- Pardi DS, Tremaine WJ, Carrasco-Labra A (2016). "American Gastroenterological Association Institute Technical Review on the Medical Management of Microscopic Colitis" (PDF). Gastroenterology. 150 (1): 247–274.e11. doi:10.1053/j.gastro.2015.11.006. PMID 26584602.
- British national formulary : BNF 58 (58 ed.). British Medical Association. 2009. pp. 56–57. ISBN 9780857111562.
- Domeij, Bengt (2000). Pharmaceutical patents in Europe. The Hague: Kluwer Law International. p. 278. ISBN 9789041113481. Archived from the original on 8 December 2015.
- Hamley, Peter (2015). Small Molecule Medicinal Chemistry: Strategies and Technologies. John Wiley & Sons. p. 390. ISBN 9781118771693. Archived from the original on 8 December 2015.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 451. ISBN 9781284057560.
- "Budesonide". International Drug Price Indicator Guide. Retrieved 5 December 2015.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Budesonide - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2011. Available at http://www.ginasthma.org Archived 2013-10-14 at the Wayback Machine
- Lichtenstein GR, Hanauer SB, Sandborn WJ (2009). "Management of Crohn's Disease in Adults". Am J Gastroenterol. 104 (2): 465–83. doi:10.1038/ajg.2008.168. PMID 19174807.
- Kuenzig ME, Rezaie A, Seow CH, Otley AR, Steinhart AH, Griffiths AM, et al. (2014). "Budesonide for maintenance of remission in Crohn's disease". Cochrane Database Syst Rev. 8 (8): CD002913. doi:10.1002/14651858.CD002913.pub3. PMID 25141071.
- Habal FM, Huang VW (2012). "Review Article: A Decision-Making Algorithm For the Management of Pregnancy in the Inflammatory Bowel Disease Patient". Aliment Pharmacol Ther. 35 (5): 501–15. doi:10.1111/j.1365-2036.2011.04967.x. PMID 22221203.
- Pardi, Darrell (2016). "American Gastroenterological Association Institute Technical Review on the Medical Management of Microscopic Colitis". www.gastrojournal.org.
- Stanaland, BE (April 2004). "Once-daily budesonide aqueous nasal spray for allergic rhinitis: a review". Clinical Therapeutics. 26 (4): 473–92. doi:10.1016/s0149-2918(04)90050-1. PMID 15189745.
- Rawla, P; Sunkara, T; Thandra, KC; Gaduputi, V (December 2018). "Efficacy and Safety of Budesonide in the Treatment of Eosinophilic Esophagitis: Updated Systematic Review and Meta-Analysis of Randomized and Non-Randomized Studies". Drugs in R&D. 18 (4): 259–269. doi:10.1007/s40268-018-0253-9. PMC 6277325. PMID 30387081.
- UK Drug Information
- Budesonide - Nasal Aerosol Inhaler (Rhinocort) side effects, medical uses, and drug interactions Archived November 6, 2008, at the Wayback Machine
- "Budesonide: CMDh scientific conclusions and grounds for variation, amendments to the product information and timetable for the implementation - PSUSA/00000449/201604" (PDF). European Medicines Agency (EMA). 10 March 2017. Archived (PDF) from the original on 8 September 2017. Retrieved 19 May 2017.
- Todd GR, Acerini CL, Buck JJ, Murphy NP, Ross-Russell R, Warner JT, McCance DR (2002). "Acute Adrenal Crisis in Asthmatics Treated With High-Dose Fluticasone Propionate". Eur Respir J. 19 (6): 1207–9. doi:10.1183/09031936.02.00274402. PMID 12108877.
- Todd GR, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D (2002). "Survey of Adrenal Crisis Associated With Inhaled Corticosteroids in the United Kingdom". Arch Dis Child. 87 (6): 457–61. doi:10.1136/adc.87.6.457. PMC 1755820. PMID 12456538.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 186, 1011. ISBN 978-1-4757-2085-3. Archived from the original on 8 September 2017.
- Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1253–. ISBN 978-0-7817-6879-5. Archived from the original on 8 September 2017.
External links
- "Budesonide". Drug Information Portal. U.S. National Library of Medicine.
- "Budesonide Nasal Spray". MedlinePlus.
- "Budesonide Oral Inhalation". MedlinePlus.