Whiting event
A whiting event is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or sediment disturbance.[1][2][3] The phenomenon gets its name from the white, chalky color it imbues to the water. These events have been shown to occur in temperate waters as well as tropical ones, and they can span for hundreds of meters.[3] They can also occur in both marine and freshwater environments.[4] The origin of whiting events is debated among the scientific community, and it is unclear if there is a single, specific cause. Generally, they are thought to result from either bottom sediment re-suspension or by increased activity of certain microscopic life such as phytoplankton.[5][6][1] Because whiting events affect aquatic chemistry, physical properties, and carbon cycling, studying the mechanisms behind them holds scientific relevance in various ways.[7][2][8][9][10]
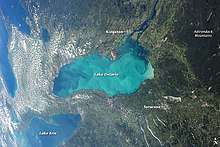
Characteristics
Whiting event clouds consist of calcium carbonate polymorphs; aragonite tends to be the dominant precipitate, but some studies in oligotrophic and mesotrophic lakes show calcite is favored.[3][7] Whiting events have been observed in tropical and temperate waters, and they can potentially cover hundreds of meters.[3] They tend to occur more often in summer months, as warmer waters promote calcium carbonate precipitation, and in hard waters.[3][10] Whitings are typically characterized by cloudy, white patches of water, but they can also be tanner in hue in very shallow waters (less than 5m deep).[2] These shallow water whiting events also tend to last less than a day in comparison to deeper water events that can last for several days up to several months.[2] Regardless of the event's lifespan, the clouds it produces increase turbidity and hamper light penetration.[10]
Potential causes
Some debate exists surrounding the exact cause of whiting events. And although much research exists on the subject, there is still no definitive consensus on the chemical mechanisms behind it. The three most common suggested causes for the phenomenon are: microbiological processes, re-suspension of marine or bottom sediments, and spontaneous direct precipitation from water.[11][3][2] Of these three, the last has been ruled unlikely due to the unfavorable reaction kinetics of spontaneous calcium carbonate precipitation.[2] It is also worth noting that it may be possible for more than one of the aforementioned factors to contribute to whiting events in the same region.[11]
Microbiological activity
Substantial findings indicate photosynthetic picoplankton, picocyanobacteria, and phytoplankton activity creates favorable conditions for carbonate precipitation.[3][2][7] This link arises as a result of planktonic blooms being observed coinciding with the events.[2][7] Subsequently, via photosynthesis, these organisms uptake inorganic carbon, raise water pH, and alter water alkalinity, which promotes calcium carbonate precipitation.[2][7] The thermodynamic influence of inorganic carbon on whiting calcium carbonate production is shown in the equation below. Furthermore, cases exist in which the type of calcium carbonate found in the whiting cloud matches the type found on local cyanobacteria membranes.[4] It's hypothesized that the extracellular polymeric substances (EPS) these microorganisms produce can act as seed crystals that provide a start for the precipitation process.[2][7] Current research on the specifics of these EPS and the exact physiological mechanisms of the microorganisms' carbon uptake, however, are limited.[2][7]
Sediment re-suspension
In shallower waters, evidence supports that activity of local fisherman and marine life such as fish and certain shark species can disturb bottom sediments containing calcium carbonate particles and lead to their suspension.[2] In addition, as microorganisms impact water chemistry in observable ways and require certain nutrient levels to thrive, whiting events found occurring in nutrient-poor waters where no significant alkalinity difference exists between whiting and non-whiting waters support the idea of sediment re-suspension as a primary cause.[12]
Relevance
Whiting events have a unique effect on the waters around them. The fact that calcium carbonate clouds increase turbidity and light reflectance holds implications for organisms and processes that depend on light.[4] In addition, whiting events can function as a transport mechanism for organic carbon to the benthic zone, which is relevant to nutrient cycling.[13] The cyanobacteria abundant clouds also hold the potential to act as a means to study the microorganism's role in carbon cycling (especially in relation to climate change) and their possible role in finding petroleum source rocks.[9][8]
References
- "Whiting Event, Lake Ontario". NASA Earth Observatory. 2 September 2013.
- Larson, Erik B.; Mylroie, John E. (2014). "A review of whiting formation in the Bahamas and new models". Carbonates and Evaporites. 29 (4): 337–347. doi:10.1007/s13146-014-0212-7. ISSN 0891-2556.
- Sondi, Ivan; Juračić, Mladen (2010). "Whiting events and the formation of aragonite in Mediterranean Karstic Marine Lakes: new evidence on its biologically induced inorganic origin". Sedimentology. 57 (1): 85–95. Bibcode:2010Sedim..57...85S. doi:10.1111/j.1365-3091.2009.01090.x. ISSN 1365-3091.
- Long, Jacqueline S.; Hu, Chuanmin; Robbins, Lisa L.; Byrne, Robert H.; Paul, John H.; Wolny, Jennifer L. (2017). "Optical and biochemical properties of a southwest Florida whiting event". Estuarine, Coastal and Shelf Science. 196: 258–268. Bibcode:2017ECSS..196..258L. doi:10.1016/j.ecss.2017.07.017. ISSN 0272-7714.
- Thompson, Joel B.; Schultze-Lam, Susanne; Beveridge, Terrance J.; Des Marais, David J. (1997). "Whiting events: Biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton". Limnology and Oceanography. 42 (1): 133–41. Bibcode:1997LimOc..42..133S. doi:10.4319/lo.1997.42.1.0133. PMID 11541205.
- "Whiting in Lake Michigan". NASA Earth Observatory. 18 September 2001.
- Dittrich, Maria; Obst, Martin (2004). "Are Picoplankton Responsible for Calcite Precipitation in Lakes?". AMBIO: A Journal of the Human Environment. 33 (8): 559–564. doi:10.1579/0044-7447-33.8.559. ISSN 0044-7447. PMID 15666689.
- Shinn, Eugene A.; St.C. Kendall, Christopher G. (2011-12-01). Day-Stirrat, Ruarri; Janson, Xavier; Wright, Wayne (eds.). "Back to the Future". The Sedimentary Record. 9 (4): 4–9. doi:10.2110/sedred.2011.4.4.
- Yates, K.K; Robbins, L.L. (2001). "Microbial Lime-Mud Production and Its Relation to Climate Change". AAPG Studies in Geology. Tulsa, Ok: American Association of Petroleum Geologists. pp. 267–283.
- Effler, Steven W.; Perkins, Mary Gail; Greer, Harry; Johnson, David L. (1987). "Effect of "whiting" on optical properties and turbidity in Owasco Lake, New York". Journal of the American Water Resources Association. 23 (2): 189–196. Bibcode:1987JAWRA..23..189E. doi:10.1111/j.1752-1688.1987.tb00796.x. ISSN 1093-474X.
- Long, Jacqueline S.; Hu, Chuanmin; Wang, Mengqiu (February 2018). "Long-term spatiotemporal variability of southwest Florida whiting events from MODIS observations". International Journal of Remote Sensing. 39 (3): 906–923. Bibcode:2018IJRS...39..906L. doi:10.1080/01431161.2017.1392637. ISSN 0143-1161.
- Morse, John W.; Gledhill, Dwight K.; Millero, Frank J. (2003). "Caco3 precipitation kinetics in waters from the great Bahama bank". Geochimica et Cosmochimica Acta. 67 (15): 2819–2826. doi:10.1016/S0016-7037(03)00103-0.
- Hodell, David A.; Schelske, Claire L. (1998). "Production, sedimentation, and isotopic composition of organic matter in Lake Ontario". Limnology and Oceanography. 43 (2): 200–214. Bibcode:1998LimOc..43..200H. doi:10.4319/lo.1998.43.2.0200. ISSN 0024-3590.
Further reading
- Thompson, Joel B.; et al. (1997). "Whiting Event: Biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton" (PDF). American Society of Limnology and Oceanography. pp. 133–141.
- Effler, Steven W.; Perkins, Mary Gail; Greer, Harry; Johnson, David L. (1987). "Effect of 'whiting' on Optical Properties and Turbidity in Owasco Lake, New York". Journal of the American Water Resources Association. 23 (2): 189–96. Bibcode:1987JAWRA..23..189E. doi:10.1111/j.1752-1688.1987.tb00796.x.
- "Whiting Events(Calcium Carbonate Precipitate)A naturally occurring phenomena" (PDF).