Wall-associated kinase
Wall-associated kinases (WAKs) are one of many classes of plant proteins known to serve as a medium between the extracellular matrix (ECM) and cytoplasm of cell walls. They are serine-threonine kinases that contain epidermal growth factor (EGF) repeats, a cytoplasmic kinase and are located in the cell walls.[1] They provide a linkage between the inner and outer surroundings of cell walls.[2] WAKs are under a group of receptor-like kinases (RLK) that are actively involved in sensory and signal transduction pathways especially in response to foreign attacks by pathogens[3] and in cell development.[4] On the other hand, pectins are an abundant group of complex carbohydrates present in the primary cell wall that play roles in cell growth and development, protection, plant structure and water holding capacity.
| Wall-associated kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
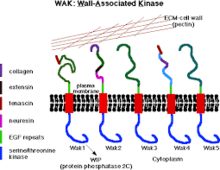 WAKs and Pectin Within The Cell Wall | |||||||||
| Identifiers | |||||||||
| Symbol | WAK | ||||||||
| Pfam | PF08488 | ||||||||
| InterPro | IPR013695 | ||||||||
| Membranome | 725 | ||||||||
| |||||||||
Pectins are rich in galacturonic acids (OGs) and present in the middle lamellae in plant tissues where they provide strength, flexibility and adhesion between plant cells.[5] Commercially and within the food industry, they are used as gels and stabilizers for desserts and juices. The role of WAKs in cell walls as pectin receptors is vital to a variety of functions involved with cell differentiation, form and host-pathogen relations.[6]
WAKs bind pectin
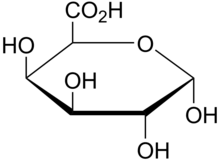
Wall-associated kinases are receptors with a calcium mediated cross-link to the cell wall of plants.[7][8] The presence of a galacturonic acid backbone in the various types of pectin is predicted to be a vital feature for binding to WAKs as WAK1 and WAK2 bind to different pectins including polymers of homogalacturonan (HA) the most abundant pectin in cell walls;[9] Oligogalacturonic acids (OG), and to rhamnogalacturonans (RG) I and II.[10] In-vitro binding between WAKs and pectin is facilitated by charged oxygen groups on pure pectin fragments and charged residues on the ECM of WAKs.[11]
Pectinase, an enzyme responsible for degrading pectin present in the cell wall releases WAKs, this became the primary suggestion that WAKs are bound to pectin within the cell wall.[10] Additionally, this hypothesis suggested a covalent bond between pectin and WAKs as they are still bound to each other after exposure to the detergent Sodium Dodecyl Sulfate (a detergent) and Dithiothreitol (DTT) and in acrylamide gels.[2] Pectin methyl-esterases (PMEs) remove methyl groups arising from the enzyme which polymerizes pectins (methyl esterified α- (1–4) D-galacturonic acid polymer) resulting in a de-esterified pectin polymer.[12] WAKs bind more readily to de-esterified pectins due to their more negative charge. This suggestion that charge is responsible for the preferable binding of WAKs to de-esterified pectins (negatively charged) was shown in a mutation in cationic residues in a WAK1 gene to neutral residues which resulted to the loss of binding properties to the de-esterified pectins.[2]
This role of charge in binding is further proved through a substitution of arginine residues for glutamine and lysine for threonines within the ECM that showing a reduced binding to the de-esterified pectin.[11] De-esterification of pectins is therefore a need for the activation of WAKs.
Molecular interactions of WAKs and pectin
Model
The binding of WAKs to pectins trigger the functioning of several pathways. Fragmentation of pectins (oligogalacturonic acid) during wounding or pathogenic attack results in a plant stress response, and WAKs play a role in the mediation of that response. However, since WAKs are also required for cell growth by binding to long pectin polymers for plant development and also pectin fragments for wounding response, no means has been found as to how WAKs differentiates between the two types of pectins to either initiate cell elongation or protection.[7] However, a model was proposed to demonstrate the preference of WAKs for de-esterified pectins and a possible explanation for initiating pathogen response rather than growth response.
A dominant WAKs allele that requires a pectin binding domain and kinase activity was shown to induce a stress response, however, this allele was suppressed with a null allele of pectin methyl-esterase (pme) which prevented the removal of the methyl groups that polymerize pectin to a de-esterified polymer hence resulting in an esterified pectin. Since WAKs is bound more loosely in esterified pectins, more was present to bind oligogalacturonic acids (in this mutant) thereby inducing a pathogen stress response rather than a growth response.[7] WAKs dependent activation of a cell expansion pathway includes the activation of MPK3, while a pathogen response shows the activation of both MPK3 and MPK6.[10]
WAK1 and WAK2 are the most expressed protein variants of WAKS out of the five WAKs known in Arabidopsis, however WAK1 is expressed most in the vasculature while WAK2 is also expressed in organ junctions, abscission zones and meristems.[8]
WAK1: pathogen response
A pathogen’s path to infection begins with the cell wall; the proteins that connect the cell wall to the plasma membrane are the initial mediators in the pathogen response. WAK1 is induced in a plant’s pathogenic response along with other pathogen-related proteins that function in protection. Wak1 is present in Arabidopsis plant tissue, with WAK1 mRNA expression more abundant in plant stem, leaves than in the roots and its extracellular domain contains epidermal growth repeats that facilitates cell signaling. Heat and salt do not have an effect in WAK1 production within tissues, however wounding is significant as it causes the expression of a WAK1 message by 2,2-dichloroisonicotinic acid (INA), a natural salicylate (SA) in the signal transduction pathway in the plant response to infection. Since WAK1 is vital to the survival of a plant in response to pathogens, it simultaneously confers resistant to SA to a point where the plant can survive exposure to high levels of SA.[13] Increased resistance to SA through WAK1 expression can only be conferred through ectopic expression of an entire WAK1 protein or kinase domain.[13] This ultimately means that inducing WAK1 expression causes decreased SA levels and a reduced toxicity, hence protection, demonstrating a role of WAK1 in regulating pathogenic attacks.
WAK2: pectin and gene expression
Pectin affects the expression of WAK2 dependent genes such as those involved in cell wall integrity and external response;[11][7] WAK2 is suggested to be important in cellular events and gene expression in Arabidopsis mesophyll. Gene expression using Affymetrix expression arrays with RNA from wild-type or wak2-1 (null mutation) protoplasts treated or not treated with pectin reveals a variety of things. In pectin-treated wild type protoplasts, there was a change in the expression of over 200 genes, with almost 50 of the upregulated genes being those involved in cell-wall synthesis such as pectin esterase, leucine-rich transmembrane kinase, plant defensin. The remainder of the downregulated genes comprised those involved in multiple functions through the plant; however, only one gene in the pectin-treated WAK2-1 was differentially expressed. In comparison to wak2-1, 13 out of the 50 upregulated genes in the wild-type was suppressed in wak2-1 and 37 were expressed similarly to the wild type. 20 genes within those downregulated showed reduced expression in wak2-1 cells, 24 were activated and the remainder had levels similar to the wild type.[11]
These patterns allowed identification of genes regulated by WAK2 without pectin treatment, those independent of WAK2 but dependent in the WAK2 pectin response. WAK2 expression in wak2-1 (null mutation) showed the greatest reduction in expression indicating that the gene was not transcribed . WAK1 and WAK2 were upregulated in pectin-treated wild-types but this was not observed in the wak2-1.[11] Evidently, WAK2 is an important component of the pectin signaling pathway, as the absence of WAK2 can amply reduce the transcriptional response to pectin. Both upregulated and downregulated WAK2-dependent pectin-response genes are either related to defense, cell wall structure, protein phosphorylation related or transcription factors.[11]
References
- Kohorn BD (October 2001). "WAKs; cell wall associated kinases". Current Opinion in Cell Biology. 13 (5): 529–33. doi:10.1016/S0955-0674(00)00247-7. PMID 11544019.
- Wagner TA, Kohorn BD (February 2001). "Wall-associated kinases are expressed throughout plant development and are required for cell expansion". The Plant Cell. 13 (2): 303–18. doi:10.1105/tpc.13.2.303. PMC 102244. PMID 11226187.
- Yang K, Qi L, Zhang Z (2014). "Isolation and characterization of a novel wall-associated kinase gene TaWAK5 in wheat (Triticum aestivum)". The Crop Journal. 2 (5): 255–266. doi:10.1016/j.cj.2014.04.010.
- Receptor-like Kinases in Plants. Signaling and Communication in Plants. 13. SpringerLink. 2012. doi:10.1007/978-3-642-23044-8. ISBN 978-3-642-23043-1.
- Willats WG, McCartney L, Mackie W, Knox JP (2001). Plant Cell Walls. Springer, Dordrecht. pp. 9–27. doi:10.1007/978-94-010-0668-2_2. ISBN 9789401038614.
- Decreux A, Messiaen J (February 2005). "Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation". Plant & Cell Physiology. 46 (2): 268–78. doi:10.1093/pcp/pci026. PMID 15769808.
- Kohorn BD (2015-08-07). "The state of cell wall pectin monitored by wall associated kinases: A model". Plant Signaling & Behavior. 10 (7): e1035854. doi:10.1080/15592324.2015.1035854 (inactive 2020-01-22). PMC 4622591. PMID 26251881.
- Kohorn BD (January 2016). "Cell wall-associated kinases and pectin perception". Journal of Experimental Botany. 67 (2): 489–94. doi:10.1093/jxb/erv467. PMID 26507892.
- Voragen, Alphons G. J.; Coenen, Gerd-Jan; Verhoef, René P.; Schols, Henk A. (2009-04-01). "Pectin, a versatile polysaccharide present in plant cell walls". Structural Chemistry. 20 (2): 263. doi:10.1007/s11224-009-9442-z.
- Kohorn BD, Kohorn SL (2012). "The cell wall-associated kinases, WAKs, as pectin receptors". Frontiers in Plant Science. 3: 88. doi:10.3389/fpls.2012.00088. PMC 3355716. PMID 22639672.
- Kohorn BD, Johansen S, Shishido A, Todorova T, Martinez R, Defeo E, Obregon P (December 2009). "Pectin activation of MAP kinase and gene expression is WAK2 dependent". The Plant Journal. 60 (6): 974–82. doi:10.1111/j.1365-313x.2009.04016.x. PMC 3575133. PMID 19737363.
- Kohorn BD (January 2016). "Cell wall-associated kinases and pectin perception". Journal of Experimental Botany. 67 (2): 489–94. doi:10.1093/jxb/erv467. PMID 26507892.
- He Z, He D, Kohorn BD (1998-04-01). "Requirement for the induced expression of a cell wall associated receptor kinase for survival during the pathogen response". The Plant Journal. 14 (1): 55–63. doi:10.1046/j.1365-313x.1998.00092.x. PMID 9681026.