4-Vinylcyclohexene
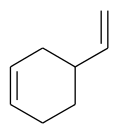
4-Vinylcyclohexene is an organic compound consisting of a vinyl group attached to the 4-position of the cyclohexene ring. It is a colorless liquid. Although chiral, it is used mainly as the racemate. It is a precursor to vinylcyclohexene dioxide.[4]
 | |
| Names | |
|---|---|
| IUPAC name
4-Vinylcyclohexene | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.590 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H12 | |
| Molar mass | 108.184 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.8299 g/cm3 at 20°C |
| Melting point | −108.9 °C (−164.0 °F; 164.2 K) |
| Boiling point | 128.9 °C (264.0 °F; 402.0 K) |
| 0.05 g/L[1] | |
| Solubility | soluble in benzene, diethyl ether, petroleum ether |
| Vapor pressure | 2 kPa |
Refractive index (nD) |
1.4639 (20 °C) |
| Hazards | |
| Safety data sheet | Oxford University |
| R-phrases (outdated) | R20 R22 |
| NFPA 704 (fire diamond) | |
| Flash point | 21.2 °C (70.2 °F; 294.3 K) [2] |
| 269 °C (516 °F; 542 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2563 mg/kg (oral, rat)[3] |
| Related compounds | |
Related compounds |
1,3-Butadiene Cyclohexene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
It is produced by 1,3-butadiene dimerizes in a Diels-Alder reaction.[5][4] The reaction is conducted at 110 - 425 °C at pressures of 1.3 - 100 MPa in the presence of a catalyst. A mixture of silicon carbide and salts of copper or chromium comprises the catalyst. A competing product is 1,5-cyclooctadiene.
Safety
4-Vinylcyclohexene is classified as a Group 2B carcinogen by the IARC ("possibly carcinogenic to humans").[2]
References
- Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 8–111. ISBN 0-8493-0594-2.
- "4-Vinylcyclohexene" (PDF). IARC. Retrieved 2009-04-19.
- "Safety (MSDS) data for 4-vinylcyclohexene". Oxford University. Retrieved 2009-04-19.
- Schiffer, Thomas; Oenbrink, Georg. "Cyclododecatriene, Cyclooctadiene, and 4-Vinylcyclohexene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_205.pub2.
- Wittcoff, Harold; Reuben, B. G.; Plotkin, Jeffrey S. (1998). Industrial Organic Chemicals (2 ed.). Wiley-Interscience. pp. 236–7. ISBN 978-0-471-44385-8. Retrieved 2009-04-19.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.

