Tropospheric ozone depletion events
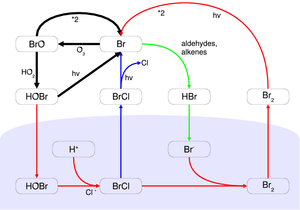
During springtime in the polar regions, unique photochemistry converts inert halide salt ions (e.g. Br−) into reactive halogen species (e.g. Br atoms and BrO) that episodically deplete ozone in the atmospheric boundary layer to near zero levels. Since their discovery in the late 1980s, research on these ozone depletion events (ODEs) has shown the central role of bromine photochemistry. Due to the autocatalytic nature of the reaction mechanism, it has been called bromine explosion. It's still not fully understood how salts are transported from the ocean and oxidized to become reactive halogen species in the air. Other halogens (chlorine and iodine) are also activated through mechanisms coupled to bromine chemistry. The main consequence of halogen activation is chemical destruction of ozone, which removes the primary precursor of atmospheric oxidation, and generation of reactive halogen atoms/oxides that become the primary oxidizing species. The different reactivity of halogens as compared to OH and ozone has broad impacts on atmospheric chemistry, including near complete removal and deposition of mercury, alteration of oxidation fates for organic gases, and export of bromine into the free troposphere. Recent changes in the climate of the Arctic and state of the Arctic sea ice cover are likely to have strong effects on halogen activation and ODEs.
See also
- Arctic haze
- Free radical halogenation
- Tropospheric ozone
- Frost flower (sea ice)