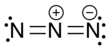Trinitrogen
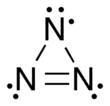
Trinitrogen also known as the azide radical is an unstable molecule composed of three nitrogen atoms. Two arrangements are possible: a linear form with double bonds and charge transfer, and a cyclic form. Both forms are highly unstable. More-stable derivatives exist, such as when it acts as a ligand, and it may participate in azido nitration, which is a reaction between sodium azide and ammonium cerium nitrate.[1][2]
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Azide radical | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChEBI |
| ||
| ChemSpider |
| ||
| 770 | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| N3 | |||
| Molar mass | 42.021 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The linear form of N3 was discovered in 1956 by B. A. Thrush[3] by photolysis of hydrogen azide.[4] As a linear and symmetric molecule, it has D∞h symmetry, with a nitrogen–nitrogen bond length averaging 1.8115 Å. The first excited electronic state, A2Σu, is 4.56 eV above the ground state.[5]
The cyclic form was identified in 2003 by N. Hansen and A. M. Wodtke using ultraviolet photolysis of chlorine azide. Although the reaction yielded mostly the linear form, about 20% of the molecules were cyclic.[3][5]
References
- Schlegel, H. Bernhard; Skancke, Anne (August 1993). "Thermochemistry, energy comparisons, and conformational analysis of hydrazine, triazane, and triaminoammonia". Journal of the American Chemical Society. 115 (16): 7465–7471. doi:10.1021/ja00069a053.
- Inorganic Molecules. Landolt-Börnstein - Group II Molecules and Radicals. 25A. 1998. doi:10.1007/b59072. ISBN 3-540-61713-2.
- Jin, Lin; Yu, Xue-fang; Pang, Jing-lin; Zhang, Shao-wen; Ding, Yi-hong (30 July 2009). "Theoretical Study on the Reactions of the Cyclic Trinitrogen Radical toward Oxygen and Water". The Journal of Physical Chemistry A. 113 (30): 8500–8505. Bibcode:2009JPCA..113.8500J. doi:10.1021/jp810741v.
- Thrush, B. A. (10 April 1956). "The Detection of Free Radicals in the High Intensity Photolysis of Hydrogen Azide". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 235 (1200): 143–147. Bibcode:1956RSPSA.235..143T. doi:10.1098/rspa.1956.0071.
- Hansen, N.; Wodtke, A. M. (December 2003). "Velocity Map Ion Imaging of Chlorine Azide Photolysis: Evidence for Photolytic Production of Cyclic-N3". The Journal of Physical Chemistry A. 107 (49): 10608–10614. Bibcode:2003JPCA..10710608H. doi:10.1021/jp0303319.
External links