Triisopropylamine
Triisopropylamine is an organic chemical compound consisting of three isopropyl groups bound to a central nitrogen atom.[1][2] As a hindered tertiary amine, it can be used as a non-nucleophilic base and as a stabilizer for polymers; however, its applications are limited by its relatively high cost and difficult synthesis.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Di(propan-2-yl)propan-2-amine | |
| Other names
Tri(propan-2-yl)amine (Triisopropyl)amine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.020.289 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H21N | |
| Molar mass | 143.274 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Ichtyal, ammoniacal |
| Density | 0.752 g/cm3 |
| Boiling point | 131.8 °C (269.2 °F; 404.9 K) 47°C at 1.9 kPa |
| Related compounds | |
Related amines |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
Triisopropylamine is notable as being among the most sterically hindered amines currently known. The even more crowded tri-tert-butylamine (tBu3N) has never been synthesized, although ab initio quantum chemical computations as well as the existence of the even more crowded 2,2,4,4-tetramethyl-3-t-butyl-pentane-3-ol (tri-tert-butylcarbinol, tBu3COH) implies that it should be a stable molecule if it could be prepared. To date, di-tert-butyl(isopropyl)amine (tBu2iPrN) has been prepared in low yield, as have a handful of tri-tert-alkylamines in which two of the tert-alkyl groups are tied together in a ring, but the authors of a 2018 study predict that tBu3N will likely remain a longstanding unsolved synthetic challenge.[3]
In the early 1990s, theoretical studies and electron diffraction analysis of the 3D structure of the molecule, in the gas phase or in non-polar solvents, indicated that the bonds between the nitrogen atom and the three carbon atoms were nearly coplanar in the ground state, instead of forming a trigonal pyramid as in simpler amines.[4][5] The average C-N-C angle was claimed to be 119.2°,[2] much closer to the 120° of the flat configuration than to the 111.8° of trimethylamine. This peculiarity was attributed to steric hindrance by the bulky isopropyl radicals. However, in 1998 X-ray diffraction analysis of the crystallized solid showed that the C3N core is actually pyramidal, with the N atom lying approximately 0.28 Å off the carbons' plane (whereas in trimethylamine the distance is about 0.45 Å). However the researchers could not rule out the crystal field effect as the cause of the asymmetry.[6]
The C-C-C planes of the isopropyl groups are slightly tilted (about 5°) relative to the threefold symmetry axis of the C3N core.[4][6][7]
Preparation
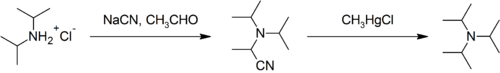
Steric effects make triisopropylamine difficult to synthesise and unlike less hindered tertiary amines (such as triethylamine) it cannot be produced by the alkylation of ammonia with alcohol; attempts to do so stall at diisopropylamine. It can be prepared from diisopropylamine on the laboratory scale:[2]
Industrial synthesis involves the reaction of ammonia with propylene oxide followed by hydrogenation.[8]
See also
References
- G. Graner, E. Hirota, T. Iijima, K. Kuchitsu, D. A. Ramsay, J. Vogt and N. Vogt (2003), C9H21N, Triisopropylamine. In Molecules Containing Five or More Carbon Atoms, volume 25D of the series Landolt-Börnstein - Group II Molecules and Radicals. Springer-Verlag. ISBN 978-3-540-42860-2; DOI 10.1007/10735542_789.
- Hans Bock; Ilka Goebel; Zdenek Havlas; Siegfried Liedle; Heinz Oberhammer (1991). "Triisopropylamine: A Sterically Overcrowded Molecule with a Flattened NC3 Pyramid and a "p-Type" Nitrogen Electron Pair". Angew. Chem. Int. Ed. 30 (2): 187–190. doi:10.1002/anie.199101871.
- Banert, Klaus; Heck, Manuel; Ihle, Andreas; Kronawitt, Julia; Pester, Tom; Shoker, Tharallah (2018-05-04). "Steric Hindrance Underestimated: It is a Long, Long Way to Tri- tert -alkylamines". The Journal of Organic Chemistry. 83 (9): 5138–5148. doi:10.1021/acs.joc.8b00496. ISSN 0022-3263.
- Arthur M. Halpern; B. R. Ramachandran (1992). "Photophysics of a sterically crowded tertiary-saturated amine: triisopropylamine". J. Phys. Chem. 96 (24): 9832–9839. doi:10.1021/j100203a047.
- Christoph Kölmel, Christian Ochsenfeld & Reinhart Ahlrichs (1992). "An ab initio investigation of structure and inversion barrier of triisopropylamine and related amines and phosphines". Theoretical Chemistry Accounts: Theory, Computation, and Modeling (Theoretica Chimica Acta). 82 (3–4).
- Boese, R.; Bläser, D.; Antipin, M. Y.; Chaplinski, V.; de Meijere, A. (1998). "Non-planar structures of Et3N and Pri3N: a contradiction between the X-ray, and NMR and electron diffraction data for Pri3N". Chem. Commun. (7): 781–782. doi:10.1039/a708399h.
- Yang M, Albrecht-Schmitt T, Cammarata V, Livant P, Makhanu DS, Sykora R, Zhu W (2009). "Trialkylamines more planar at nitrogen than triisopropylamine in the solid state". J. Org. Chem. 74 (7): 2671–8. doi:10.1021/jo802086h. PMID 19323571.
- Sk A3 932005, "Wasteless process for preparing triisopropanolamine", issued Dec 5, 2008, assigned to Novacke Chemicke Zavody