Thioketene
Thioketenes are organosulfur compounds analogous to ketenes with the general formula R2C=C=S, where R is alkyl or aryl. Thioketene (ethenthione) is also the name of the compound CH2=C=S, which is the simplest thioketene. Thioketenes are reactive, tending to polymerize. Some thioketenes are produced as transient species upon pyrolysis of 1,2,3-thiadiazoles.[1]
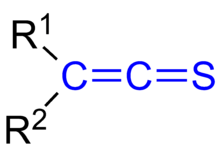
The general structure of thioketenes
Bis(trifluoromethyl)thioketene ((CF3)2C=C=S) is a rare example of a stable thioketene.[2] Another stable thioketene is carbon subsulfide (S=C=C=C=S).
References
- Otto-Albrecht Neuman (Editor). Rompps Encyclopedia of Chemistry, Frank'sche Publishing House, Stuttgart, 1983, 8. Edition, p. 4242, ISBN 3-440-04513-7.
- Raasch, Maynard S. (1970). "Bis(trifluoromethyl)thioketene. I. Synthesis and cycloaddition reactions". J. Org. Chem. 35: 3470–3483. doi:10.1021/jo00835a064.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.