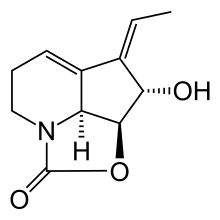
Streptazolin
Streptazolin is an antibiotic and antifungal substance isolated in 1981 from Streptomyces viridochromogenes.[1][2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2a1S,6Z,7S,7aS)-6-ethyliden-2a1,3,4,6,7,7a-hexahydro-7-hydroxy-1-oxa-2a-azacyclopenta[cd]inden-2-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C11H13NO3 | |
| Molar mass | 207.229 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Because of its polymerisation tendency, it is not suitable for therapeutic use. 1,4-reduction of the conjugated diene gives dihydrostreptazolin which is stable, but has very limited antimicrobial properties.[1]
The first total synthesis of (racemic) streptazolin was achieved in 1985 with the aid of a modified Ferrier rearrangement.[3][4]
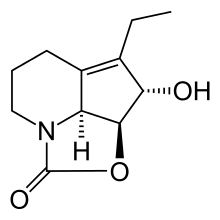
(+)-Dihydrostreptazolin
References
- Drautz H, Zähner H (1981). "Isolation and structure of streptazolin". Helv. Chim. Acta. 64 (6): 1752–65. doi:10.1002/hlca.19810640605.
- Karrer A, Dobler M (1982). "Stoffwechselprodukte von Mikroorganismen 217. Mitteilung Röntgenstrukturanalyse von O-Acetyldihydrostreptazolin". Helv. Chim. Acta. 65 (5): 1432–35. doi:10.1002/hlca.19820650516.
- Kozikowski AP, Pyeong-uk Park (1984). "Synthesis of 2-substituted .DELTA.3-piperidines: the nitrogen analog of the Ferrier rearrangement. An approach to streptazolin". J. Org. Chem. 49 (9): 1674–1676. doi:10.1021/jo00183a044.
- Kozikowski AP,((Pyeong-uk Park)) (1985). "Total synthesis of streptazolin - an application of the aza-analogue of the ferrier rearrangement". J. Am. Chem. Soc. 107 (6): 1763–65. doi:10.1021/ja00292a054.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.