Secondary carbon
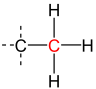
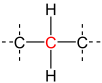
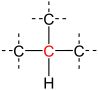
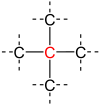
A secondary carbon is a carbon atom bound to two other carbon atoms.[1] For this reason, secondary carbon atoms are found in all hydrocarbons having at least three carbon atoms. In unbranched alkanes, the inner carbon atoms are always secondary carbon atoms (see figure).[2]
| primary carbon | secondary carbon | tertiary carbon | quaternary carbon | |
| General structure (R = Organyl group) |
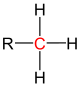 |
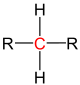 |
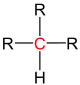 |
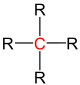 |
| Partial Structural formula |
 |
 |
 |
 |
| secondary Carbon |
|---|
_V1.svg.png) |
| Structural formula of propane (secondary carbon is highlighted red) |
References
- Smith, Janice Gorzynski (2011). "Chapter 4 Alkanes". Organic chemistry (Book) (3rd ed.). New York, NY: McGraw-Hill. p. 116. ISBN 978-0-07-337562-5.
- Hans Peter Latscha, Uli Kazmaier, Helmut Alfons Klein (2016), Organische Chemie: Chemie-Basiswissen II (in German) (7. Auflage ed.), Berlin: Springer Spektrum, p. 40, ISBN 978-3-662-46180-8CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.