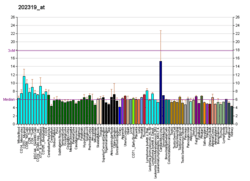
SENP6
Sentrin-specific protease 6 is an enzyme that in humans is encoded by the SENP6 gene.[5]
Function
Ubiquitin-like molecules (UBLs), such as SUMO1, are structurally related to ubiquitin and can be ligated to target proteins in a similar manner as ubiquitin. However, covalent attachment of UBLs does not result in degradation of the modified proteins. SUMO1 modification is implicated in the targeting of RANGAP1 to the nuclear pore complex, as well as in stabilization of I-kappa-B-alpha (NFKBIA; MIM 164008) from degradation by the 26S proteasome. Like ubiquitin, UBLs are synthesized as precursor proteins, with 1 or more amino acids following the C-terminal glycine-glycine residues of the mature UBL protein. Thus, the tail sequences of the UBL precursors need to be removed by UBL-specific proteases, such as SENP6, prior to their conjugation to target proteins
See also
References
- GRCh38: Ensembl release 89: ENSG00000112701 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000034252 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: SENP6 SUMO1/sentrin specific peptidase 6".
Further reading
- Mikolajczyk J, Drag M, Békés M, Cao JT, Ronai Z, Salvesen GS (September 2007). "Small ubiquitin-related modifier (SUMO)-specific proteases: profiling the specificities and activities of human SENPs". The Journal of Biological Chemistry. 282 (36): 26217–24. doi:10.1074/jbc.M702444200. PMID 17591783.
- Drag M, Mikolajczyk J, Krishnakumar IM, Huang Z, Salvesen GS (January 2008). "Activity profiling of human deSUMOylating enzymes (SENPs) with synthetic substrates suggests an unexpected specificity of two newly characterized members of the family". The Biochemical Journal. 409 (2): 461–9. doi:10.1042/BJ20070940. PMID 17916063.
- Yeh ET, Gong L, Kamitani T (May 2000). "Ubiquitin-like proteins: new wines in new bottles". Gene. 248 (1–2): 1–14. doi:10.1016/S0378-1119(00)00139-6. PMID 10806345.
- Nagase T, Ishikawa K, Suyama M, Kikuno R, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O (October 1998). "Prediction of the coding sequences of unidentified human genes. XI. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Research. 5 (5): 277–86. doi:10.1093/dnares/5.5.277. PMID 9872452.
- Kim KI, Baek SH, Jeon YJ, Nishimori S, Suzuki T, Uchida S, Shimbara N, Saitoh H, Tanaka K, Chung CH (May 2000). "A new SUMO-1-specific protease, SUSP1, that is highly expressed in reproductive organs". The Journal of Biological Chemistry. 275 (19): 14102–6. doi:10.1074/jbc.275.19.14102. PMID 10799485.
- Goodson ML, Hong Y, Rogers R, Matunis MJ, Park-Sarge OK, Sarge KD (May 2001). "Sumo-1 modification regulates the DNA binding activity of heat shock transcription factor 2, a promyelocytic leukemia nuclear body associated transcription factor". The Journal of Biological Chemistry. 276 (21): 18513–8. doi:10.1074/jbc.M008066200. PMID 11278381.
- Tojo M, Matsuzaki K, Minami T, Honda Y, Yasuda H, Chiba T, Saya H, Fujii-Kuriyama Y, Nakao M (November 2002). "The aryl hydrocarbon receptor nuclear transporter is modulated by the SUMO-1 conjugation system". The Journal of Biological Chemistry. 277 (48): 46576–85. doi:10.1074/jbc.M205987200. PMID 12354770.
- Bailey D, O'Hare P (January 2004). "Characterization of the localization and proteolytic activity of the SUMO-specific protease, SENP1". The Journal of Biological Chemistry. 279 (1): 692–703. doi:10.1074/jbc.M306195200. PMID 14563852.
- Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villén J, Li J, Cohn MA, Cantley LC, Gygi SP (August 2004). "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proceedings of the National Academy of Sciences of the United States of America. 101 (33): 12130–5. doi:10.1073/pnas.0404720101. PMC 514446. PMID 15302935.
- Choi SJ, Chung SS, Rho EJ, Lee HW, Lee MH, Choi HS, Seol JH, Baek SH, Bang OS, Chung CH (October 2006). "Negative modulation of RXRalpha transcriptional activity by small ubiquitin-related modifier (SUMO) modification and its reversal by SUMO-specific protease SUSP1". The Journal of Biological Chemistry. 281 (41): 30669–77. doi:10.1074/jbc.M604033200. PMID 16912044.
- Mukhopadhyay D, Ayaydin F, Kolli N, Tan SH, Anan T, Kametaka A, Azuma Y, Wilkinson KD, Dasso M (September 2006). "SUSP1 antagonizes formation of highly SUMO2/3-conjugated species" (PDF). The Journal of Cell Biology. 174 (7): 939–49. doi:10.1083/jcb.200510103. PMC 2064386. PMID 17000875.
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (November 2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.