Rothemund reaction
The Rothemund reaction is a condensation/oxidation process that converts four pyrroles and four aldehydes into a porphyrin. It is based on work by Paul Rothemund, who first reported it in 1936.[1][2] His techniques underpin more modern synthesis such as those described by Adler and Longo.[3] In solution-phase synthesis, acidic conditions are essential; formic acid, acetic acid, and propionic acid typical reaction solvents, or p-toluenesulfonic acid or various Lewis acids can be used with a non-acidic solvent. A large amount of side-product is formed and is removed, usually by recrystallization or chromatography.

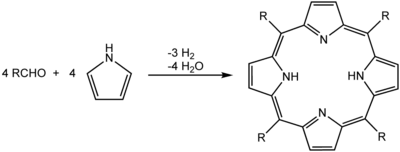
Green chemistry variants have been developed in which the reaction is performed with microwave irradiation using reactants adsorbed on acidic silica gel[4] or at high temperature in the gas phase.[5] In these cases, no additional acid is required.
The synthesis of simple porphyrins such as meso-tetraphenylporphyrin (H2TPP) is also commonly done in university teaching labs.[6]
References
- P. Rothemund (1936). "A New Porphyrin Synthesis. The Synthesis of Porphin". J. Am. Chem. Soc. 58 (4): 625–627. doi:10.1021/ja01295a027.
- P. Rothemund (1935). "Formation of Porphyrins from Pyrrole and Aldehydes". J. Am. Chem. Soc. 57 (10): 2010–2011. doi:10.1021/ja01313a510.
- A. D. Adler; F. R. Longo; J. D. Finarelli; J. Goldmacher; J. Assour; L. Korsakoff (1967). "A simplified synthesis for meso-tetraphenylporphine". J. Org. Chem. 32 (2): 476. doi:10.1021/jo01288a053.
- Petit, A.; Loupy, A.; Maiuard, P.; Momenteau, M. (1992). "Microwave Irradiation in Dry Media: A New and Easy Method for Synthesis of Tetrapyrrolic Compounds". Synth. Commun. 22 (8): 1137–1142. doi:10.1080/00397919208021097.
- Drain, C. M.; Gong, X. (1997). "Synthesis of meso substituted porphyrins in air without solvents or catalysts". Chem. Commun. (21): 2117–2118. doi:10.1039/A704600F.
- Falvo, RaeAnne E.; Mink, Larry M.; Marsh, Diane F. (1999). "Microscale Synthesis and 1H NMR Analysis of Tetraphenylporphyrins". J. Chem. Educ. 1999 (76): 237–239. Bibcode:1999JChEd..76..237M. doi:10.1021/ed076p237.