Reduction of nitro compounds
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
Aromatic nitro compounds
Reduction to anilines

The reduction of nitroaromatics is conducted on an industrial scale.[1] Many methods exist, such as:
- Catalytic hydrogenation using: Raney nickel[2] or palladium-on-carbon,[3] platinum(IV) oxide, or Urushibara nickel[4].
- Iron in acidic media.[5] The reaction conditions are typically gentle. Iron has a high functional group tolerance (see Bechamp reduction).
- Sodium hydrosulfite[6]
- Sodium sulfide (or hydrogen sulfide and base). Illustrated by the selective reduction of dinitrophenol to the nitroaminophenol.[7]
- Tin(II) chloride[8]
- Titanium(III) chloride
- Samarium[9]
- Hydroiodic acid[10]
Metal hydrides are typically not used to reduce aryl nitro compounds to anilines because they tend to produce azo compounds. (See below)
Reduction to hydroxylamines
Several methods have been described for the production of aryl hydroxylamines from aryl nitro compounds:
- Raney nickel and hydrazine at 0-10 °C[11]
- Electrolytic reduction[12]
- Zinc metal in aqueous ammonium chloride[13]
Reduction to hydrazino compounds
Treatment of nitroarenes with excess zinc metal results in the formation of N,N'-diarylhydrazine.[14]
Reduction to azo compounds

Treatment of aromatic nitro compounds with metal hydrides gives good yields of azo compounds. For example, one could use:
- Lithium aluminium hydride[15]
- Zinc metal with sodium hydroxide.[14] (Excess zinc will reduce the azo group to a hydrazino compound.)
Aliphatic nitro compounds
Reduction to hydrocarbons

Hydrodenitration (replacement of a nitro group with hydrogen) is difficult to achieve but can be effected by catalytic hydrogenation over platinum on silica gel at high temperatures.[16] The reaction can also be effected through radical reaction with tributyltin hydride and a radical initiator, AIBN as an example.[17]
Reduction to amines
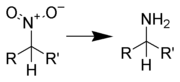
Aliphatic nitro compounds can be reduced to aliphatic amines by several reagents:
- Catalytic hydrogenation using platinum(IV) oxide (PtO2)[18] or Raney nickel[19]
- Iron metal in refluxing acetic acid[20]
- Samarium diiodide[21]
- Raney nickel, platinum on carbon, or zinc dust and formic acid or ammonium formate[4]
α,β-Unsaturated nitro compounds can be reduced to saturated amines by:
- Catalytic hydrogenation over palladium-on-carbon
- Iron metal
- Lithium aluminium hydride[22] (Note: Hydroxylamines and oximes are typical impurities.)
- Lithium borohydride or sodium borohydride and trimethylsilyl chloride[23]
- Red-Al[24]
Reduction to hydroxylamines
Aliphatic nitro compounds can be reduced to aliphatic hydroxylamines using diborane.[25]
The reaction can also be carried out with zinc dust and ammonium chloride :
- R-NO2 + 4 NH4Cl + 2 Zn → R-NH-OH + 2 ZnCl2 + 4 NH3 + H2O
Reduction to oximes

Nitro compounds are typically reduced to oximes using metal salts, such as stannous chloride[26] or chromium(II) chloride.[27] Additionally, catalytic hydrogenation using a controlled amount of hydrogen can generate oximes.[28]
References
- Gerald Booth (2007). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411.
- Allen, C. F. H.; VanAllan, J. (1955). "2-Amino-p-cymene". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 3, p. 63
- Bavin, P. M. G. (1973). "2-Aminofluorene". Organic Syntheses.; Collective Volume, 5, p. 30
- Adams, J. P. (2002). "Nitro and related groups". Journal of the Chemical Society, Perkin Transactions 1. 0 (23): 2586–2597. doi:10.1039/b009711j.
- Fox, B. A.; Threlfall, T. L. (1964). "2,3-Diaminopyridine". Organic Syntheses. 44: 34. doi:10.15227/orgsyn.044.0034.CS1 maint: multiple names: authors list (link)
- Redemann, C. T.; Redemann, C. E. (1955). "5-Amino-2,3-dihydro-1,4-phthalazinedione". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 3, p. 69
- Hartman, W. W.; Silloway, H. L. (1945). "2-Amino-4-nitrophenol". Organic Syntheses. 25: 5. doi:10.15227/orgsyn.025.0005.
- Faul, Margaret M.; Thiel, Oliver R. (2005). "Tin(II) Chloride". Encyclopedia of Reagents for Organic Synthesis. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt112.pub2. ISBN 9780470842898.
- Basu, M. K. (2000). "Ultrasound-promoted highly efficient reduction of aromatic nitro compounds to the aromatic amines by samarium/ammonium chloride". Tetrahedron Lett. 41 (30): 5603–5606. doi:10.1016/S0040-4039(00)00917-5.
- Kumar, J. S. Dileep; Ho, ManKit M.; Toyokuni, Tatsushi (2001). "Simple and chemoselective reduction of aromatic nitro compounds to aromatic amines: reduction with hydriodic acid revisited". Tetrahedron Letters. 42 (33): 5601–5603. doi:10.1016/s0040-4039(01)01083-8.
- Ayyangar, N. R.; Brahme, K. C.; Kalkote, U. R.; Srinivasan, K. V. (1984). "Facile Transfer-Reduction of Nitroarenes to N Arylhydroxylamines with Hydrazine in the Presence of Raney Nickel". Synthesis. 1984 (11): 938. doi:10.1055/s-1984-31027.
- Harman, R. E. (1963). "Chloro-p-benzoquinone". Organic Syntheses.; Collective Volume, 4, p. 148
- Kamm, O. (1941). "β-Phenylhydroxylamine". Organic Syntheses.; Collective Volume, 1, p. 445
- Bigelow, H. E.; Robinson, D. B. (1955). "Azobenzene". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 3, p. 103
- R. F. Nystrom & W. G. Brown (1948). "Reduction of Organic Compounds by Lithium Aluminum Hydride. III. Halides, Quinones, Miscellaneous Nitrogen Compounds". J. Am. Chem. Soc. 70 (11): 3738–3740. doi:10.1021/ja01191a057. PMID 18102934.
- M. J. Guttieri & W. F. Maier (1984). "Selective cleavage of carbon-nitrogen bonds with platinum". J. Org. Chem. 49 (16): 2875–2880. doi:10.1021/jo00190a006.
- T. V. (Babu) RajanBabu, Philip C. Bulman Page, Benjamin R. Buckley, "Tri-n-butylstannane" Encyclopedia of Reagents for Organic Synthesis 2004, John Wiley & Sons. doi:10.1002/047084289X.rt181.pub2
- A. T. Nielsen (1962). "The Isomeric Dinitrocyclohexanes. II. Stereochemistry". J. Org. Chem. 27 (6): 1998–2001. doi:10.1021/jo01053a019.
- Dauben, Jr., H. J.; Ringold, H. J.; Wade, R. H.; Pearson, D. L.; Anderson, Jr., A. G. (1963). "Cycloheptanone". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 4, p. 221
- Senkus, M. (1948). "Ind. Eng. Chem". 40: 506. Cite journal requires
|journal=(help) - A. S. Kende & J. S. Mendoza (1991). "Controlled reduction of nitroalkanes to alkyl hydroxylamines or amines by samarium diiodide". Tetrahedron Letters. 32 (14): 1699–1702. doi:10.1016/S0040-4039(00)74307-3.
- A. Burger, M. L. Stein and J. B. Clements (1957). "Some Pyridylnitroalkenes, Nitroalkanols, and Alkylamines". J. Org. Chem. 22 (2): 143–144. doi:10.1021/jo01353a010.
- Giannis, A.; Sandhoff, K. (1989). "LiBH4(NaBH4)/Me3SiCl, an Unusually Strong and Versatile Reducing Agent". Angewandte Chemie International Edition in English. 28 (2): 218–220. doi:10.1002/anie.198902181.
- Butterick, John R.; Unrau, A. M. (1974). "Reduction of β-nitrostyrene with sodium bis-(2-methoxyethoxy)-aluminium dihydride. A convenient route to substituted phenylisopropylamines". J. Chem. Soc., Chem. Commun. 0 (8): 307–308. doi:10.1039/c39740000307.
- H. Feuer, R. S. Bartlett, B. F. Vincent and R. S. Anderson (1965). "Diborane Reduction of Nitro Salts. A New Synthesis of N-Monosubstituted Hydroxylamines". J. Org. Chem. 30 (9): 2880–2882. doi:10.1021/jo01020a002.CS1 maint: multiple names: authors list (link)
- Braun, V. J.; Sobecki, W. (1911). "Über primäre Dinitro-, Nitronitrit- und Dialdoxim-Verbindungen der Fettreihe". Ber. 44 (3): 2526–2534. doi:10.1002/cber.19110440377.
- J. R. Hanson & E. Premuzic (1967). "Applications of chromous chloride--II : The reduction of some steroidal nitro-compounds". Tetrahedron. 23 (10): 4105–4110. doi:10.1016/S0040-4020(01)97921-9.
- C. Grundmann (1950). "Über die partielle Reduktion von Nitro-cyclohexan". Angewandte Chemie. 62 (23–24): 558–560. doi:10.1002/ange.19500622304.
