Quinhydrone electrode
The quinhydrone electrode may be used to measure the hydrogen ion concentration (pH) of a solution containing an acidic substance.[1][2]
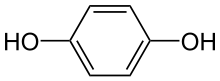
hydroquinone

quinone
A platinum wire electrode is immersed in a saturated aqueous solution of quinhydrone, in which there is the following equilibrium
- C
6H
6O
2 ⇌ C
6H
4O
2 + 2H+ +2e−.
The potential difference between the platinum electrode and a reference electrode is dependent on the activity, , of hydrogen ions in the solution.
The quinhydrone electrode provides an alternative to the most commonly used glass electrode.[3] however, it is not reliable above pH 8 and cannot be used with solutions that contain a strong oxidizing or reducing agent.[1]
References
- Bates, Roger G. Determination of pH: theory and practice. Wiley, 1973, pp 246-252
- Rossotti, F. J. C.; Rossotti, H. (1961). The Determination of Stability Constants. McGraw-Hill., p 135
- Vonau, W.; Guth, U (2006). "pH Monitoring: a review". Journal of Solid State Electrochemistry. 10 (9): 746–752. doi:10.1007/s10008-006-0120-4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.