Pyridinium
Pyridinium refers to the cation [C5H5NH]+. It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.[3]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
pyridinium | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H6N | |||
| Molar mass | 80.110 g·mol−1 | ||
| Acidity (pKa) | ~5 (for the conjugate acid)[1][2] | ||
| Conjugate base | Pyridine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
As pyridine is often used as an organic base in chemical reactions, pyridinium salts are produced in many acid-base reactions. Its salts are often insoluble in the organic solvent, so precipitation of the pyridinium leaving group complex is an indication of the progress of the reaction. The pyridinium ion also plays a role in Friedel-Crafts acylation. When pyridine is included, it forms a complex with the electrophilic acylium ion, rendering it even more reactive.
Pyridinium cations are aromatic ion, as determined through Hückel's rule.[4] It is isoelectronic to benzene.
N-Alkylpyridinium cations
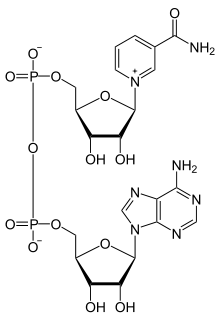
When the acidic proton is replaced by alkyl, the compounds are called N-alkylpyridinium. A simple representative is N-methylpyridinium ([C5H5NCH3]+). From a commercial perspective, an important pyridinium compound is the herbicide paraquat.[5]
References
- Linnell, Robert (1960). "Notes – Dissociation Constants of 2-Substituted Pyridines". Journal of Organic Chemistry. 25 (2): 290. doi:10.1021/jo01072a623.
- Pearson, Ralph G.; Williams, Forrest V. (1953). "Rates of Ionization of Pseudo Acids.1V. Steric Effects in the Base-catalyzed Ionization of Nitroethane". Journal of the American Chemical Society. 75 (13): 3073. doi:10.1021/ja01109a008.
- George A. Olah, Michael Watkins (1978). "Fluorinations With Pyridinium Polyhydrogen Fluoride Reagent: 1-Fluoroadamantane". Org. Synth. 58: 75. doi:10.15227/orgsyn.058.0075.CS1 maint: uses authors parameter (link)
- "Aromatic Compounds" (PDF). Alex Roche, Rutgers University.
- Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.