Pyramidal inversion
In chemistry, pyramidal inversion is a fluxional process in compounds with a pyramidal molecule, such as ammonia (NH3) "turns inside out".[1][2] It is a rapid oscillation of the atom and substituents, the molecule or ion passing through a planar transition state.[3] For a compound that would otherwise be chiral due to a stereocenter, pyramidal inversion allows its enantiomers to racemize.
Energy barrier
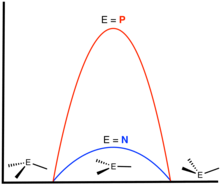
The identity of the inverting atom has a dominating influence on the barrier. Inversion of ammonia is rapid at room temperature. In contrast, phosphine (PH3) inverts very slowly at room temperature (energy barrier: 132 kJ/mol).[4] Consequently, amines of the type RR′R"N usually are not optically stable (enantiomers racemize rapidly at room temperature), but P-chiral phosphines are.[5] Appropriately substituted sulfonium salts, sulfoxides, arsines, etc. are also optically stable near room temperature. Steric effects can also influence the barrier.
References
- Arvi Rauk, Leland C. Allen, Kurt Mislow (1970). "Pyramidal Inversion". Angew. Chem. Int. Ed. 9: 400–414. doi:10.1002/anie.197004001.CS1 maint: uses authors parameter (link)
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Pyramidal inversion". doi:10.1351/goldbook.P04956
- J. M. Lehn (1970). "Nitrogen Inversion: Experiment and Theory". Fortschr. Chem. Forsch. 15: 311–377. doi:10.1007/BFb0050820.
- Kölmel, C.; Ochsenfeld, C.; Ahlrichs, R. (1991). "An ab initio investigation of structure and inversion barrier of triisopropylamine and related amines and phosphines". Theor. Chim. Acta. 82 (3–4): 271–284. doi:10.1007/BF01113258.
- Xiao, Y.; Sun, Z.; Guo, H.; Kwon, O. (2014). "Chiral Phosphines in Nucleophilic Organocatalysis". Beilstein Journal of Organic Chemistry. 10: 2089–2121. doi:10.3762/bjoc.10.218. PMC 4168899.CS1 maint: uses authors parameter (link)