Purpurogallin
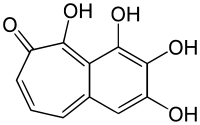
Purpurogallin is a red, crystalline compound, and the aglycone of several glycosides from nutgalls and oak barks.[1] It can inhibit 2-hydroxy and 4-hydroxyestradiol methylation by catechol-O-methyltransferase.[2] It potently and specifically inhibits TLR1/TLR2 activation pathway.[3]
 | |
| Names | |
|---|---|
| IUPAC name
2,3,4,5-Tetrahydroxybenzo[7]annulen-6-one | |
| Other names
Purpurogalline 2,3,4,6-Tetrahydroxybenzocyclohepten-5-one PPG | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.478 |
| KEGG | |
| MeSH | C026133 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H8O5 | |
| Molar mass | 220.180 g·mol−1 |
| Appearance | Red crystalline solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Wu, Tai-Wing; Zeng, Ling-Hua; Wu, Jun; Fung, Kwok-Pui; Weisel, Richard D; Hempel, Andrew; Camerman, Norman (1996). "Molecular structure and antioxidant specificity of purpurogallin in three types of human cardiovascular cells". Biochemical Pharmacology. 52 (7): 1073. doi:10.1016/0006-2952(96)00447-9. PMID 8831727.
- Lambert, Joshua D; Chen, Dapeng; Wang, Ching Y; Ai, Ni; Sang, Shengmin; Ho, Chi-Tang; Welsh, William J; Yang, Chung S (2005). "Benzotropolone inhibitors of estradiol methylation: Kinetics and in silico modeling studies". Bioorganic & Medicinal Chemistry. 13 (7): 2501. doi:10.1016/j.bmc.2005.01.037.
- Cheng, Kui; Wang, Xiaohui; Zhang, Shuting; Yin, Hang (2012). "Discovery of Small-Molecule Inhibitors of the TLR1/TLR2 Complex". Angewandte Chemie International Edition. 51 (49): 12246. doi:10.1002/anie.201204910. PMC 3510333. PMID 22969053.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.