Primary biliary cholangitis
Primary biliary cholangitis (PBC), previously known as primary biliary cirrhosis, is an autoimmune disease of the liver.[1][2][3] It results from a slow, progressive destruction of the small bile ducts of the liver, causing bile and other toxins to build up in the liver, a condition called cholestasis. Further slow damage to the liver tissue can lead to scarring, fibrosis, and eventually cirrhosis.
| Primary biliary cholangitis | |
|---|---|
| Other names | Primary biliary cirrhosis |
 | |
| Micrograph of PBC showing bile duct inflammation and injury. H&E stain. | |
| Specialty | Gastroenterology |
| Symptoms | Cholestasis, pruritus, fatigue |
| Complications | Cirrhosis, hepatic failure, portal hypertension |
| Usual onset | Usually middle-aged women |
| Causes | Autoimmune |
| Diagnostic method | Anti-mitochondrial antibodies, liver biopsy |
Common symptoms are tiredness, itching and, in more advanced cases, jaundice. In early cases, there may only be changes in blood tests.[4]
PBC is a relatively rare disease, affecting up to 1 in 3,000–4,000 people.[5][6] It is much more common in women, with a sex ratio of at least 9:1 female to male.[1]
The condition has been recognised since at least 1851 and was named "primary biliary cirrhosis" in 1949.[7] Because cirrhosis is a feature only of advanced disease, a change of its name to "primary biliary cholangitis" was proposed by patient advocacy groups in 2014.[8][9]
Signs and symptoms
People with PBC experience fatigue (80 percent): this is a non-specific symptom; it can be debilitating, with a huge impact on quality of life. Its pathogenesis is still unknown and it is quite challenging to explore its specificity and to treat. Comorbidities that could contribute or worse fatigue, such as depression, hypothyroidism, anaemia, obesity, or medication side effects, should be promptly identified and treated. Dry skin and dry eyes are also common. Itching (pruritus) occurs in 20–70 percent.[4] Pruritus can develop at any stage of the disease, it does not correlate with progression of liver disease, and may even improve or disappear as disease gets more advanced. It is usually reported by over 70% of patients, and it is typically mild-to-moderate in intensity. Given the impact on quality of life and night sleep, pruritus is correlated with fatigue. It can rarely be severe, non-responsive to medical therapy and requiring liver transplant. Pruritus is characteristically intermittent, worse at night, and improves during summer. People with more severe PBC may have jaundice (yellowing of the eyes and skin).[4] PBC impairs bone density and there is an increased risk of fracture.[4] Xanthelasma (skin lesions around the eyes) or other xanthoma may be present as a result of increased cholesterol levels.[10]
PBC can eventually progress to cirrhosis of the liver. This in turn may lead to a number of symptoms or complications:
- Fluid retention in the abdomen (ascites) in more advanced disease
- Enlarged spleen in more advanced disease
- Oesophageal varices in more advanced disease
- Hepatic encephalopathy, including coma in extreme cases in more advanced disease.
People with PBC may also sometimes have the findings of an associated extrahepatic autoimmune disorder such as thyroid disease or rheumatoid arthritis or Sjögren's syndrome (in up to 80 percent of cases).[10][11]
Causes
PBC has an immunological basis, and is classified as an autoimmune disorder. It results from a slow, progressive destruction of the small bile ducts of the liver, with the intralobular ducts and the Canals of Hering (intrahepatic ductules) being affected early in the disease.
Most people with PBC (>90 percent) have anti-mitochondrial antibodies (AMAs) against pyruvate dehydrogenase complex (PDC-E2), an enzyme complex that is found in the mitochondria. People who are negative for AMAs are usually found to be positive when more sensitive methods of detection are used.[12]
People with PBC may also have been diagnosed with another autoimmune disease, such as a rheumatological, endocrinological, gastrointestinal, pulmonary, or dermatological condition, suggesting shared genetic and immune abnormalities.[11] Common associations include Sjögren's syndrome, systemic sclerosis, rheumatoid arthritis, lupus, hypothyroidism and coeliac disease.[13][14]
A genetic predisposition to disease has been thought to be important for some time. Evidence for this includes cases of PBC in family members, identical twins both having the condition (concordance), and clustering of PBC with other autoimmune diseases. In 2009, a Canadian-led group of investigators reported in the New England Journal of Medicine results from the first PBC genome-wide association study.[15][16] This research revealed parts of the IL12 signaling cascade, particularly IL12A and IL12RB2 polymorphisms, to be important in the aetiology of the disease in addition to the HLA region. In 2012, two independent PBC association studies increased the total number of genomic regions associated to 26, implicating many genes involved in cytokine regulation such as TYK2, SH2B3 and TNFSF11.[17][18]
A study of over 2000 patients identified a gene - POGLUT1 - that appeared to be associated with this condition.[19] Earlier studies have also suggested that this gene may be involved. The implicated protein is an endoplasmic reticulum O-glucosyltransferase.
An environmental Gram negative alphabacterium — Novosphingobium aromaticivorans[20] has been associated with this disease with several reports suggesting an aetiological role for this organism.[21][22][23] The mechanism appears to be a cross reaction between the proteins of the bacterium and the mitochondrial proteins of the liver cells. The gene encoding CD101 may also play a role in host susceptibility to this disease.[24]
There is a failure of immune tolerance against the mitochondrial pyruvate dehydrogenase complex (PDC-E2), and this may also be the case with other proteins, including the gp210 and p62 nuclear pore proteins. Gp210 has increased expression in the bile duct of anti-gp210 positive patients, and these proteins may be associated with prognosis.[25]
Clinical presentation and diagnosis
Most patients are currently diagnosed when asymptomatic, having been referred to the hepatologist for abnormal liver function tests (mostly raised GGT or alkaline phosphatase [ALP]) performed for annual screening blood tests. Other frequent scenarios include screening of patients with non-liver autoimmune diseases, e.g. rheumatoid arthritis, or investigation of elevated cholesterol, evaluation of itch or unresolved cholestasis post-partum. Diagnosing PBC is generally straightforward. The basis for a definite diagnosis are reported below:
- Abnormalities in liver enzyme tests are usually present and elevated gamma-glutamyl transferase and alkaline phosphatase (ALP) are found in early disease.[10] Elevations in bilirubin occur in advanced disease.
- Antimitochondrial antibodies are the characteristic serological marker for PBC, being found in 90-95 percent of patients and only 1 percent of controls. PBC patients have AMA against pyruvate dehydrogenase complex (PDC-E2), an enzyme complex that is found in the mitochondria.[10] Those people who are AMA negative but with disease similar to PBC have been found to have AMAs when more sensitive detection methods are employed.[12]
- Other auto-antibodies may be present:
- Antinuclear antibody measurements are not diagnostic for PBC because they are not specific, but may have a role in prognosis.
- Anti-glycoprotein-210 antibodies, and to a lesser degree anti-p62 antibodies, correlate with the disease's progression toward end stage liver failure. Anti-gp210 antibodies are found in 47 percent of PBC patients.[26][27]
- Anti-centromere antibodies often correlate with developing portal hypertension.[28]
- Anti-np62[29] and anti-sp100 are also found in association with PBC.
- Abdominal ultrasound, MR scanning (MRCP) or a CT scan is usually performed to rule out blockage to the bile ducts. This may be needed if a condition causing secondary biliary cirrhosis, such as other biliary duct disease or gallstones, needs to be excluded. A liver biopsy may help, and if uncertainty remains as in some patients, an endoscopic retrograde cholangiopancreatography (ERCP), an endoscopic investigation of the bile duct, may be performed.
Given the high specificity of serological markers, liver biopsy is not necessary for the diagnosis of PBC; however, it is still necessary when PBC-specific antibodies are absent, or when co-existent autoimmune hepatitis (AIH) or non-alcoholic steatohepatitis (NASH) is suspected. Liver biopsy can be useful to stage the disease for fibrosis and ductopenia. Finally, it may also be appropriate in the presence of other extra-hepatic comorbidities.
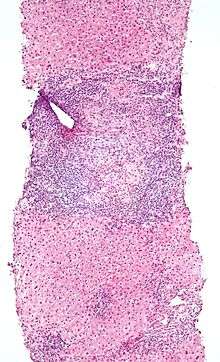 Low magnification micrograph of PBC. H&E stain.
Low magnification micrograph of PBC. H&E stain. Intermediate magnification micrograph of PBC showing bile duct inflammation and periductal granulomas. Liver biopsy. H&E stain.
Intermediate magnification micrograph of PBC showing bile duct inflammation and periductal granulomas. Liver biopsy. H&E stain.
Liver biopsy
On microscopic examination of liver biopsy specimens, PBC is characterized by chronic, non-suppurative inflammation, which surrounds and destroys interlobular and septal bile ducts. These histopathologic findings in primary biliary cholangitis include the following:[30]
- Inflammation of the bile ducts, characterized by intraepithelial lymphocytes, and
- Periductal epithelioid granulomata.
- Proliferation of bile ductules
- Fibrosis (scarring)
The Ludwig and Scheuer scoring systems have historically been used to stratify four (1–4) ‘stages’ of PBC, with stage 4 indicating the presence of cirrhosis. In the new system of Nakanuma, the stage of disease is based on fibrosis, bile duct loss and features of cholate-stasis, i.e. deposition of orcein-positive granules, whereas the grade of necroinflammatory activity is based on cholangitis and interface hepatitis. The accumulation of orcein-positive granules occurs evenly across the PBC liver, which means that staging using the Nakanuma system is more reliable regarding sampling variability.
Liver biopsy for the diagnosis and staging of PBC lost favour after the evidence of a patchy distribution of the duct lesions and fibrosis across the organ. The widespread availability of non-invasive measures of fibrosis means that liver biopsy for staging of PBC is somewhat obsolete. Liver biopsy does, however, remain useful in certain settings. The main indications are to confirm the diagnosis of PBC when PBC-specific antibodies are absent and confirm a diagnosis of PBC with AIH features (i.e. overlap PBC-AIH). Liver biopsy is also useful to assess the relative contribution of each liver injury when a comorbid liver disease is present, such as non-alcoholic steatohepatitis. In patients with inadequate response to UDCA, liver biopsy may provide the explanation and could undoubtedly inform risk stratification. For example, it may identify a previously unsuspected variant syndrome, steatohepatitis, or interface hepatitis of moderate or greater severity. It is also useful in AMA and ANA-specific antibody negative cholestatic patients to indicate an alternative process, e.g. sarcoidosis, small duct PSC, adult idiopathic ductopenia.
Histopathology stages (by Ludwig and Scheuer systems)
- Stage 1 – Portal Stage: Normal sized triads; portal inflammation, subtle bile duct damage. Granulomas are often detected in this stage.
- Stage 2 – Periportal Stage: Enlarged triads; periportal fibrosis and/or inflammation. Typically characterized by the finding of a proliferation of small bile ducts.
- Stage 3 – Septal Stage: Active and/or passive fibrous septa.
- Stage 4 – Biliary Cirrhosis: Nodules present; garland or jigsaw puzzle pattern.
Treatment
Medical therapy of PBC targets disease progression and symptom control. The backbone of treatment of PBC is bile acid. UDCA has been the only drug available for two decades and more recently obeticholic acid (OCA), a semi-synthetic hydrophobic bile acid analogue, has been licensed in patients failing UDCA response or intolerant to UDCA. Several other agents have been studied, including immunosuppressants, but robust evidence of benefit is lacking.[10][31][32]
- Ursodeoxycholic acid (UDCA), marketed as Ursodiol, Ursobil and others, has been shown to improve the liver biochemistry, slow down histological progression and improve LT-free survival.[33][10] Patients with PBC who have an inadequate response to UDCA or those few (<3%) who are intolerant to UDCA should be candidates for second-line therapies.
- Obeticholic acid (OCA) is approved for patients with an inadequate response to UDCA or for patients unable to tolerate UDCA.
- Fibric acid derivatives, or fibrates, are agonists of the peroxisome proliferator activator receptor (PPAR), a nuclear receptor involved in several metabolic pathways. Fibrates are licensed for the treatment of hypertriglyceridemia. They also exert potent anticholestatic effects. Among the fibrates, bezafibrate and fenofibrate, PPAR-alpha selective agonists, have been extensively studied as therapeutic agents because of their potential ability to decrease bile acid synthesis and bile acid-related hepatic inflammation. A Randomised, controlled trial in 2018 showed its efficacy in patients with inadequate response to UDCA.
- Budesonide is currently used as off-label medication in PBC. In PBC patients showing interface hepatitis on liver biopsy some groups demonstrated the efficacy of budesonide in improving liver histology and biochemistry when used in combination with UDCA. Results of a randomised, controlled trial conclused in 2017 are awaited.
- To relieve itching caused by bile acids in circulation, which are normally removed by the liver, cholestyramine (a bile acid sequestrant) may be prescribed to absorb bile acids in the gut and be eliminated, rather than re-enter the blood stream. Other drugs that do this include rifampicin, naltrexone and sertraline.
- Fatigue is a non-specific but often reported symptom in PBC, and represents an unmet need since there are no licensed therapies. A structured approach to management, quantifying fatigue and its impacts (through the use of disease-specific tools such as the PBC-40 quality of life measures), addressing contributing and exacerbating factors and supporting patients to cope with its impact is effective. Drugs such as Coenzyme Q and Rituximab have been shown to be ineffective. A graded programme of exercise helps some individuals.
- People with PBC may have poor lipid-dependent absorption of Vitamins A, D, E, K.[34] Appropriate supplementation is recommended when bilirubin is elevated.[10]
- People with PBC are at elevated risk of developing osteoporosis[35] as compared to the general population and others with liver disease. Screening and treatment of this complication is an important part of the management of PBC.
- As in all liver diseases, consumption of alcohol should be restricted or eliminated.
- In patients with advanced liver disease the only curative therapy is liver transplant. Outcomes are favourable with 5-year patient survival rates better than for most other indications for LT (80–85%). [36][37]
Prognosis
The introduction of UDCA has dramatically changed the pattern and the course of the disease. Numerous trials and observational studies have demonstrated its efficacy on liver biochemistry, histological progression and transplant-free survival [38]
Among the UDCA treated patients, the degree of the liver biochemistry improvement, i.e. the UDCA-response, identifies patients with different long-term prognosis. LT-free survival of patients with normal or near-normal liver biochemistry on UDCA is similar to that of the general population, whereas it is significantly reduced in those with abnormal liver biochemistry on treatment.
The two most important parameters in evaluating response to UDCA are ALP and total bilirubin. Qualitative and quantitative definitions of UDCA-response have been developed, based on changes of bilirubin, transaminases and ALP, after a time frame of 6 to 24 months of treatment with UDCA at 13-15 mg/kg/day. [39]
We are today also able to risk-stratify patients at diagnosis based on the probability of UDCA-response. This is relevant in order to early identify patients who would be eligible for second-line therapies before waiting for the treatment failure under UDCA, with potential impact on disease course. [40]
HCC is infrequent in PBC. Recent large-scale cohort studies highlighted as that the lack of UDCA-response after 12 months of therapy and male sex are associated with increased future risk of developing HCC in PBC.
After liver transplant, the recurrence of disease rate may be as high as 18 percent at five years, and up to 30 percent at 10 years. There is no consensus on risk factors for recurrence of the disease.[41]
Epidemiology
Epidemiologic studies report heterogeneous incidence rates of 0.33 to 5.8 per 100.000 inhabitants per year, and prevalence rates of 1.9 to 40.2 per 100.000 inhabitants. Such figures, in particular the prevalence, have shown some increasing in the last decades. Improvement of diagnostic tools, increasing disease awareness, and digitalized patient registration with easing of case-findings, along with an improved survival likely contributed to the rising prevalence rates. The disease has been described worldwide, even though North America and Northern Europe have shown the highest incidence and prevalence rates. It is not clear whether there is a true variation in disease prevalence among populations of different geographical areas and of different ethnicity or if this is a consequence of a difference in study quality. [5] [6] PBC is more common in women, with a female:male ratio of at least 9:1. The peak incidence of PBC is in the fifth decade of life. In some areas of the US and UK, the prevalence is estimated to be as high as 1 in 4000. This is much more common than in South America or Africa, which may be due to better recognition in the US and UK. [5][6] First-degree relatives may have as much as a 500 times increase in prevalence, but there is debate if this risk is greater in the same generation relatives or the one that follows.
PBC is considered a prime example of the female preponderance in autoimmunity with a female to male ratio of up to 9:1, confirmed by large cohort studies, although some recent data, using administrative registries, suggest an increasing male prevalence. Major defects of sex chromosomes, i.e. enhanced monosomy X in female patients and an enhanced Y chromosome loss in male patients, have been described and might well explain the greater female predisposition to develop PBC. [42]
Even though there are case reports of patients diagnosed at the age of 15 or 93, the typical disease onset is between 30 and 60 years. It is estimated that prevalence of PBC in women over the age of 45 years could exceed 1 in 800 individuals.
History
The first report of the disease dates back 1851 by Addison and Gull who described a clinical picture of progressive jaundice in the absence of mechanical obstruction of the large bile ducts. Ahrens et al. in 1950 published the first detailed description of 17 patients with this condition and coined the term “primary biliary cirrhosis”. In 1959, Dame Sheila Sherlock reported a further series of PBC patients and recognised that the disease could be diagnosed in a pre-cirrhotic stage and proposed the term “chronic intrahepatic cholestasis” as more appropriate description of this disease. However, this nomenclature failed to gain acceptance and the term “primary biliary cirrhosis” lasted for decades. In 2014, to correct the inaccuracy and remove the social stigmata of cirrhosis as well as all the misunderstanding, disadvantages and discrimination emanating from this misnomer in daily life for patients, international liver associations agreed to rename the disease “primary biliary cholangitis”, as it is known nowadays.[43].[7] [44] [45]
Society and culture
Support groups
PBC Foundation
The PBC Foundation is a UK-based international charity offering support and information to people with PBC, their families and friends.[46] It campaigns for increasing recognition of the disorder, improved diagnosis and treatments, and estimates over 8000 people are undiagnosed in the UK.[47][48] The Foundation has supported research into PBC including the development of the PBC-40 quality of life measure published in 2004[49] and helped establish the PBC Genetics Study.[17][50] It was founded by Collette Thain in 1996, after she was diagnosed with the condition.[47] Thain was awarded an MBE Order of the British Empire in 2004 for her work with the Foundation.[51] The PBC Foundation helped initiate the name change campaign in 2014.[8][9][52]
PBCers Organization
The PBCers Organization is a US-based non-profit patient support group that was founded by Linie Moore in 1996 and advocates for greater awareness of the disease and new treatments.[53] It has supported the initiative for a change in name.[9]
Name
In 2014 the PBC Foundation, with the support of the PBCers Organization, the PBC Society (Canada)[54] and other patient groups, advocated a change in name from "primary biliary cirrhosis" to "primary biliary cholangitis," noting that most PBC patients did not have cirrhosis and that "cirrhosis" often had negative connotations of alcoholism.[8][9][52] Patient and professional groups were canvassed.[55] Support for the name change came from professional bodies including the American Association for the Study of Liver Diseases[56] and the European Association for the Study of the Liver.[57] Advocates for the name change published calls to adopt the new name in multiple hepatology journals in the fall of 2015.[55][57][58][59]
References
- Poupon R (2010). "Primary Biliary Cirrhosis: A 2010 Update". Journal of Hepatology. 52 (5): 745–758. doi:10.1016/j.jhep.2009.11.027. PMID 20347176.
- Hirschfield GM, Gershwin ME (January 2013). "The Immunobiology and Pathophysiology of Primary Biliary Cirrhosis". Annual Review of Pathology. 8: 303–330. doi:10.1146/annurev-pathol-020712-164014. PMID 23347352.
- Dancygier, Henryk (2010). Principles and Practice of Clinical Hepatology. Springer. pp. 895–. ISBN 978-3-642-04509-7. Retrieved 29 June 2010.
- Selmi C, Bowlus CL, Gershwin LE, Coppel RL (7 May 2011). "Primary Biliary Cirrhosis". Lancet. 377 (9777): 1600–1609. doi:10.1016/S0140-6736(10)61965-4. PMID 21529926.
- Boonstra K, Beuers U, Ponsioen CY (2012). "Epidemiology of Primary Sclerosing Cholangitis and Primary Biliary Cirrhosis: A Systematic Review". Journal of Hepatology. 56 (5): 1181–1188. doi:10.1016/j.jhep.2011.10.025. PMID 22245904.
- James OF, Bhopal R, Howel D, et al. (1999). "Primary Biliary Cirrhosis Once Rare, Now Common in the United Kingdom?". Hepatology. 30 (2): 390–394. doi:10.1002/hep.510300213. PMID 10421645.
- Dauphinee, James A.; Sinclair, Jonathan C. (July 1949). "Primary Biliary Cirrhosis". Canadian Medical Association Journal. 61 (1): 1–6. PMC 1591584. PMID 18153470.
- PBC Foundation (UK). "PBC Name Change". Retrieved 27 Jan 2017.
- PBCers Organization. "Primary Biliary Cirrhosis Name Change Initiative" (PDF).
- Lindor KD, Gershwin ME, Poupon R, et al. (July 2009). "Primary Biliary Cirrhosis". Hepatology. 50 (1): 291–308. doi:10.1002/hep.22906. PMID 19554543.
The AASLD Practice Guideline
- Floreani A, Franceschet I, Cazzagon N (August 2014). "Primary Biliary Cirrhosis: Overlaps with Other Autoimmune Disorders". Seminars in Liver Disease. 34 (3): 352–360. doi:10.1055/s-0034-1383734. PMID 25057958.
- Vierling JM (2004). "Primary Biliary Cirrhosis and Autoimmune Cholangiopathy". Clinical Liver Disease. 8 (1): 177–194. doi:10.1016/S1089-3261(03)00132-6. PMID 15062200.
- Narciso-Schiavon JL, Schiavon LL (2017). "To screen or not to screen? Celiac antibodies in liver diseases". World J Gastroenterol (Review). 23 (5): 776–791. doi:10.3748/wjg.v23.i5.776. PMC 5296194. PMID 28223722.

- Volta U, Rodrigo L, Granito A, et al. (October 2002). "Celiac Disease in Autoimmune Cholestatic Liver disorders". The American Journal of Gastroenterology. 97 (10): 2609–2613. PMID 12385447.
- Hirschfield GM, Liu X, Xu C, et al. (June 2009). "Primary Biliary Cirrhosis Associated with HLA, IL12A, and IL12RB2 Variants". The New England Journal of Medicine. 360 (24): 2544–2555. doi:10.1056/NEJMoa0810440. PMC 2857316. PMID 19458352.
- "UK-PBC – Stratified Medicine in Primary Biliary Cholangitis (PBC; formally known as Cirrhosis)".
- Liu JZ, Almarri MA, Gaffney DJ, et al. (October 2012). "Dense Fine-Mapping Study Identifies New Susceptibility Loci for Primary Biliary Cirrhosis". Nature Genetics. 44 (10): 1137–1141. doi:10.1038/ng.2395. PMC 3459817. PMID 22961000.
- Juran BD, Hirschfield GM, Invernizzi P, et al. (December 2012). "Immunochip Analyses Identify a Novel Risk Locus for Primary Biliary Cirrhosis at 13q14, Multiple Independent Associations at Four Established Risk Loci and Epistasis Between 1p31 and 7q32 Risk Variants". Human Molecular Genetics. 21 (23): 5209–5221. doi:10.1093/hmg/dds359. PMC 3490520. PMID 22936693.
- Hitomi Y, et al. (2019). "POGLUT1, the putative effector gene driven by rs2293370 in primary biliary cholangitis susceptibility locus chromosome 3q13.33". Sci Rep. 9 (1): 102. doi:10.1038/s41598-018-36490-1. PMC 6331557. PMID 30643196.
- Selmi C, Balkwill DL, Invernizzi P, et al. (November 2003). "Patients with Primary Biliary Cirrhosis React Against a Ubiquitous Xenobiotic-Metabolizing Bacterium". Hepatology. 38 (5): 1250–1257. doi:10.1053/jhep.2003.50446. PMID 14578864.
- Mohammed JP, Mattner J (July 2009). "Autoimmune Disease Triggered by Infection with Alphaproteobacteria". Expert Review of Clinical Immunology. 5 (4): 369–379. doi:10.1586/ECI.09.23. PMC 2742979. PMID 20161124.
- Kaplan MM (November 2004). "Novosphingobium aromaticivorans: a Potential Initiator of Primary Biliary Cirrhosis". The American Journal of Gastroenterology. 99 (11): 2147–2149. PMID 15554995.
- Selmi C, Gershwin ME (July 2004). "Bacteria and Human Autoimmunity: The Case of Primary Biliary Cirrhosis". Current Opinion in Rheumatology. 16 (4): 406–410. doi:10.1097/01.bor.0000130538.76808.c2. PMID 15201604.
- Mohammed JP, Fusakio ME, Rainbow DB, et al. (July 2011). "Identification of Cd101 as a Susceptibility Gene for Novosphingobium aromaticivorans-induced Liver Autoimmunity". Journal of Immunology. 187 (1): 337–349. doi:10.4049/jimmunol.1003525. PMC 3134939. PMID 21613619.
- Nakamura M, Takii Y, Ito M, et al. (March 2006). "Increased expression of Nuclear Envelope gp210 Antigen in Small Bile Ducts in Primary Biliary Cirrhosis". Journal of Autoimmunity. 26 (2): 138–145. doi:10.1016/j.jaut.2005.10.007. PMID 16337775.
- Nickowitz RE, Worman HJ (1993). "Autoantibodies From Patients With Primary Biliary Cirrhosis Recognize a Restricted Region Within the Cytoplasmic Tail of Nuclear Pore Membrane Glycoprotein Gp210". Journal of Experimental Medicine. 178 (6): 2237–2242. doi:10.1084/jem.178.6.2237. PMC 2191303. PMID 7504063.
- Bauer A, Habior A (2007). "Measurement of gp210 Autoantibodies in sera of Patients With Primary Biliary cirrhosis". Journal of Clinical Laboratory Analysis. 21 (4): 227–231. doi:10.1002/jcla.20170. PMC 6648998. PMID 17621358.
- Nakamura M, Kondo H, Mori T, et al. (January 2007). "Anti-gp210 and Anti-Centromere Antibodies Are Different Risk Factors for the Progression of Primary Biliary Cirrhosis". Hepatology. 45 (1): 118–127. doi:10.1002/hep.21472. PMID 17187436.
- Nesher G, Margalit R, Ashkenazi YJ (April 2001). "Anti-Nuclear Envelope Antibodies: Clinical Associations". Seminars in Arthritis and Rheumatism. 30 (5): 313–320. doi:10.1053/sarh.2001.20266. PMID 11303304.
- Nakanuma Y, Tsuneyama K, Sasaki M, et al. (August 2000). "Destruction of Bile Ducts in Primary Biliary Cirrhosis". Baillière's Best Practice & Research. Clinical Gastroenterology. 14 (4): 549–570. doi:10.1053/bega.2000.0103. PMID 10976014.
- Levy C, Lindor KD (April 2003). "Treatment Options for Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis". Current Treatment Options in Gastroenterology. 6 (2): 93–103. doi:10.1007/s11938-003-0010-0. PMID 12628068.
- Oo YH, Neuberger J (2004). "Options for treatment of primary biliary cirrhosis". Drugs. 64 (20): 2261–2271. doi:10.2165/00003495-200464200-00001. PMID 15456326.
- Rudic, Jelena S.; Poropat, Goran; Krstic, Miodrag N.; Bjelakovic, Goran; Gluud, Christian (2012-12-12). "Ursodeoxycholic acid for primary biliary cirrhosis". The Cochrane Database of Systematic Reviews. 12: CD000551. doi:10.1002/14651858.CD000551.pub3. ISSN 1469-493X. PMC 7045744. PMID 23235576.
- Bacon BR, O'Grady JG (2006). Comprehensive Clinical Hepatology. Elsevier Health Sciences. pp. 283–. ISBN 978-0-323-03675-7. Retrieved 29 June 2010.
- Collier JD, Ninkovic M, Compston JE (February 2002). "Guidelines on the Management of Osteoporosis Associated with Chronic Liver Disease". Gut. 50 Suppl 1 (Suppl 1): i1–9. doi:10.1136/gut.50.suppl_1.i1. PMC 1867644. PMID 11788576.
- Clavien P, Killenberg PG (2006). Medical Care of the Liver Transplant Patient: Total Pre-, Intra- and Post-Operative Management. Wiley-Blackwell. p. 155. ISBN 978-1-4051-3032-5.CS1 maint: ref=harv (link)
- Kaneko J, Sugawara Y, Tamura S, et al. (January 2012). "Long-Term Outcome of Living Donor Liver Transplantation for Primary Biliary Cirrhosis". Transplant International. 25 (1): 7–12. doi:10.1111/j.1432-2277.2011.01336.x. PMID 21923804.
- Carbone M, Mells GF, Pells G, Dawwas MF, Newton JL, Heneghan MA, Neuberger JM, Day DB, Ducker SJ, UKPBC C, Sandford RN, Alexander GJ, Jones DE (March 2013). "Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid". Gastroenterology. 144 (3): 560–569. doi:10.1053/j.gastro.2012.12.005. PMID 23246637.
- Cristoferi L, Nardi A, Ronca V, Invernizzi P, Mells G, Carbone M (December 2018). "Prognostic models in primary biliary cholangitis". Journal of Autoimmunity. 95 (1): 171–178. doi:10.1016/j.jaut.2018.10.024. PMID 30420264.
- Carbone M, Nardi A, Flack S, Carpino G, Varvaropoulou N, Gavrila C, Spicer A, Badrock J, Bernuzzi F, Cardinale V, Ainsworth HF, Heneghan MA, Thorburn D, Bathgate A, Jones R, Neuberger JM, Battezzati PM, Zuin M, Taylor-Robinson S, Donato MF, Kirby J, Mitchell-Thain R, Floreani A, Sampaziotis F, Muratori L, Alvaro D, Marzioni M, Miele L, Marra F, Giannini E, Gaudio E, Ronca V, Bonato G, Cristoferi L, Malinverno F, Gerussi A, Stocken DD, Cordell HJ, Hirschfield GM, Alexander GJ, Sandford RN, Jones DE, Invernizzi P, Mells GF, ItalianPBCStudy G, UKPBC C (September 2018). "Pretreatment prediction of response to ursodeoxycholic acid in primary biliary cholangitis: development and validation of the UDCA Response Score". Lancet Gastroenterology and Hepatology. 3 (9): 626–634. doi:10.1016/S2468-1253(18)30163-8. PMC 6962055. PMID 30017646.
- Clavien & Killenberg 2006, p. 429
- Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, Selmi C, Watnik M, Gershwin ME, Podda M (February 2004). "Frequency of monosomy X in women with primary biliary cirrhosis". Lancet. 14 (363): 533–535. doi:10.1016/S0140-6736(04)15541-4. PMID 14975617.
- Reuben A (2003). "The serology of the Addison-Gull syndrome". Hepatology. 37 (1): 225–8. doi:10.1002/hep.510370134. PMID 12500211.
- Walker JG, Doniach D, Roitt IM, Sherlock S (April 1965). "Serological Tests in Diagnosis of Primary Biliary Cirrhosis". Lancet. 1 (7390): 827–31. doi:10.1016/s0140-6736(65)91372-3. PMID 14263538.
- Mitchison HC, Bassendine MF, Hendrick A, et al. (1986). "Positive Antimitochondrial Antibody but Normal Alkaline Phosphatase: Is this Primary Biliary Cirrhosis?". Hepatology. 6 (6): 1279–1284. doi:10.1002/hep.1840060609. PMID 3793004.
- Association of Medical Research Charities. "The PBC Foundation". Archived from the original on 4 March 2016. Retrieved 12 July 2015.
- Staff of The Scotsman, January 3, 2008. Dealing with a silent killer
- Thain, Collette (2015). "Primary Biliary Cirrhosis: Getting a Diagnosis" (online). At Home Magazine. Retrieved 28 July 2015.
- Jacoby A, Rannard A, Buck D, et al. (November 2005). "Development, Validation, and Evaluation of the PBC-40, a Disease Specific Health-Related Quality of Life Measure for Primary Biliary Cirrhosis". Gut. 54 (11): 1622–1629. doi:10.1136/gut.2005.065862. PMC 1774759. PMID 15961522.
- Mells GF, Floyd JA, Morley KI, et al. (April 2011). "Genome-Wide Association study Identifies 12 New Susceptibility Loci for Primary Biliary Cirrhosis". Nature Genetics. 43 (4): 329–332. doi:10.1038/ng.789. PMC 3071550. PMID 21399635.
- Barry Gordon for The Scotsman. 31 December 2003 A royal seal of approval
- PBC Foundation. "EASL Name Change Presentation". Archived from the original on 9 July 2015. Retrieved 8 July 2015.
- Kim, Margot (2015-01-18). "New hope for PBC liver disease". ABC30 Action news. Retrieved 4 August 2015.
- Cheung AC, Montano-Loza A, Swain M, et al. (2015). "Time to Make the Change from 'Primary Biliary Cirrhosis' to 'Primary Biliary Cholangitis'". Canadian Journal of Gastroenterology and Hepatology. 29 (6): 293. doi:10.1155/2015/764684. PMC 4578449. PMID 26196152.
- Beuers U, Gershwin ME, Gish RG, et al. (2015). "Changing Nomenclature for PBC: From 'Cirrhosis' to 'Cholangitis'". Gut. 64 (11): 1671–1672. doi:10.1136/gutjnl-2015-310593. PMID 26374822.
- AASLD. "A Name Change for PBC: Cholangitis Replacing Cirrhosis". Retrieved 6 July 2015.
- Beuers U, Gershwin ME, Gish RG, et al. (2015). "Changing Nomenclature for PBC: From 'Cirrhosis' to 'Cholangitis'". Journal of Hepatology. 63 (5): 1285–1287. doi:10.1016/j.jhep.2015.06.031. PMID 26385765.
- Beuers U, Gershwin ME, Gish RG, et al. (2015). "Changing Nomenclature for PBC: From 'Cirrhosis' to 'Cholangitis'". Hepatology. 62 (5): 1620–1622. doi:10.1002/hep.28140. PMID 26372460.
- Beuers U, Gershwin ME, Gish RG, et al. (2015). "Changing Nomenclature for PBC: From 'Cirrhosis' to 'Cholangitis'". Gastroenterology. 149 (6): 1627–1629. doi:10.1053/j.gastro.2015.08.031. PMID 26385706.
External links
| Classification | |
|---|---|
| External resources |