Phasmarhabditis hermaphrodita
Phasmarhabditis hermaphrodita is a facultative parasitic nematode that can kill slugs and snails.[2] It belongs to the family Rhabditidae,[2] the same family as Caenorhabditis elegans. The Genus of Phasmarhabditis (Greek: Phasma = (φάσμα (monster); rhabditis = (ῥάβδος (rod-like)) is made up of 10 species including P. hermaphrodita,[3] P. neopapillosa,[4] P. californica,[5] P. papillosa,[5] P. apuliae,[6] P. bohemica,[7] P. bonaquaense,[8] P. huizhouensis,[9] P. nidrosiensis,[9] P. valida [9] and P. tawfiki.[10]
| Phasmarhabditis hermaphrodita | |
|---|---|
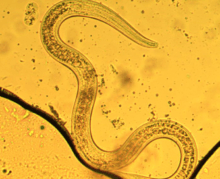 | |
| Third stage dauer larva P. hermaphrodita | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Subfamily: | Peloderinae[1] |
| Genus: | |
| Species: | P. hermaphrodita |
| Binomial name | |
| Phasmarhabditis hermaphrodita (A. Schneider, 1859) | |
P. hermaphrodita is a bacterial-feeding nematode and is a lethal parasite of several terrestrial gastropod families such as Arionidae, Milacidae and Limacidae.[2][11] It is also able to reproduce on rotting matter or penetrate and remain in resistant slug and snail species where it awaits for their death and will then reproduce on the cadaver (necromeny). P. hermaphrodita was first isolated and documented by A. Schneider in 1859 [2][11] and was intensively studied in the 1990s by researchers at Long Ashton Research centre who were focussed on finding a new biocontrol agent for slugs. P. hermaphrodita was isolated here and developed as a biological control agent (Nemaslug®) [11][12] for minimising agriculture damage from slugs and snails in 1994.
Anatomy

P. hermaphrodita is unsegmented, vermiform, bilateral symmetrical pseudocoelomate. The body dimensions and structure of P. hermaphrodita is comparable to C. elegans' with a body length 1.3 - 1.7 mm long [3] and an estimated circumference of 0.180mm. The primary structures are the Rhabditida-specific mouth, the pharynx, the intestine, the reproductive system (uterus, spermatheca, gonads) and the cuticle. Like all nematodes, it has four muscle bands that span the length of the body and has no devoted respiratory or circulatory system.[13] P. hermaphrodita has four larval stages before becoming a fully reproductive hermaphroditic adult female.[14] Males do exist in this species, but are very rare with Maupas only able to find 21 males among 15,000 individuals (0.14% of the population).[15] Third-stage dauer larvae are produced in unfavourable conditions such as low food levels, high population density or high temperatures.[16] Dauer larvae have constricted pharynx, double the thickness of a normal cuticle and increase of lipid droplets in their cytoplasm.[17] However, the most important aspect of the dauer stage is its ability to serve as the infective stage that seeks out new hosts, once the previous bacterial food source has been depleted.[18][19] Dauers do not require a food source and can survive up to eight times longer than the original life span of a non-dauer nematode.[16] P. hermaphrodita is morphologically identical to two other Phasmarhabditis species P. neopapillosa [4] and P. tawfiki.[10] However, P. neopapillosa is a gonochoristic species with an equal number of males and females.[4]
Biological control of slugs
Terrestrial gastropods are a common problem in agricultural areas with a moist climate around the world,[20] crop damage occurs via the eating of leaves and stems and/or contaminating them with slime and faeces.[21] In the UK alone, slugs affect 59% of total area for rapeseed oil crops and 22% of wheat crops.[22] Without any type slug control for both Rapeseed oil and wheat crops, the cost to the UK agricultural industry would be an approximate £43.5 million per year.[22]

P. hermaphrodita was developed into a natural molluscicide to prevent crop damage from horticultural slug pests from the families Arionidae, Milacidae, Agriolimacidae, Limacidae [12] and Vaginulidae.[23] P. hermaphrodita is the only nematode of the eight families associated with molluscs, which has been developed as a biological molluscicide, first released under the name Nemaslug® by MicroBio Ltd in 1994, then acquired by Becker Underwood in 2000 and finally taken over by BASF in 2012.[24] Nemaslug® is sold in 15 European countries and is widely used by farmers and gardeners. It can only kill slugs and snails and does not harm non-target organisms like earthworms, insects & acarids. Other methods of slug control include chemical molluscicides such as metaldehyde, iron phosphate and carbamate compounds (Methiocarb and Thiodicarb) have been shown to cause damage to many beneficial/non-beneficial non-target organisms including mammals.[25] P. hermaphrodita is currently mass produced in fermenters (up to 20,000 litres) in a monoxenic liquid broth of the bacterium Moraxella osloensis.[12] In the fields, P. hermaphrodita is applied at 3 x 109 nematodes per ha.[12] Nemaslug® has been found to be successful at reducing agricultural damage from slug in crops such as Winter wheat,[26] lettuce,[27] rapeseed,[28] strawberries, Brussels sprouts, asparagus [12] and others. Even though Nemaslug® takes longer (1–3 weeks) to kill slugs than chemical molluscicides, it has been shown to be equally or more effective at killing slugs.[29] An added advantage to P. hermaphrodita is its ability to strongly suppress feeding of infected slugs [29] and to deter non-infected slugs away from treated soil.[30]
Reproduction and Development
Phasmarhabditis hermaphrodita is protandrous autogamous hermaphrodite,[24] whose main substrate to reproduce on is mainly bacterial rich environments such as decomposing cadavers (slugs, snails, worms, insects), leaves, compost and slug faeces.[12][20] Third stage infective dauers seek out new hosts [19] responding to host cues such as slime and faeces [12][31] or a bacteria rich environment. Once a new host is found, the dauer enters the slug via the dorsal integumental pouch beneath the mantle and then to the shell cavity via the short canal,[11][19] next the dauer then develops into a self-fertilizing hermaphrodite and starts to produce young. The mother can produce up to 250–300 young whilst inside their still alive host.[24]

At this stage, depending on temperature, the weight of the gastropod, nematode density in the soil the host may die within 4 to 21 days,[11][12] however, studies show if large enough (over 1g), some slugs (e.g. Arion lusitanicus) can resist infection.[12][27] The process involved in killing the host is still not fully understood, whether it is due to internal damage from new offspring or the releasing of M. osloensis.[19] M. osloensis was found growing cultures of P. hermaphrodita [27] and was shown to kill slugs when injected in large amounts into D. reticulatum,[19] but it is not vertically transmitted to offspring, hence its role in the pathogenicity process is currently unclear.[32] When infected by P. hermaphrodita both morphological and behavioural characteristics of the slug change. Morphological changes include a swelling of the mantle area, where both fluid and reproducing nematodes accumulate.[11]
Infected slugs will also find secluded places to die, such as cracks in the soil where they can move down into and conceal themselves deeper within the soil layer.[33] This is theorised to be P. hermaphrodita manipulating the host to go to a more favourable environment. This ensures that they are left alone with the cadaver and avoid any interference with scavengers.[34] The soil also helps to prevent the cadaver from drying out, which creates a moist environment that promotes diverse bacterial growth.[35] Reproduction occurs and the next generation continues to reproduce until food runs out and more third stage infective dauers are produced and the cycle is repeated.[12]
References
- Nguyen, K. B. (3 March 2006). "SUBORDER RHABDITINA". Entomology & Nematology Department, University of Florida. Retrieved 7 December 2010.
- Genena, M. A., Mostafa, F. A., Fouly, A. H., & Yousef, A. A. (2011). First record for the slug parasitic nematode, Phasmarhabditis hermaphrodita (Schneider) in Egypt. Archives of Phytopathology and Plant Protection, 44(4), 340-345.
- De Ley, I. T., McDonnell, R. D., Lopez, S., Paine, T. D., & De Ley, P. (2014). Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae), a potential biocontrol agent isolated for the first time from invasive slugs in North America. Nematology, 16(10), 1129-1138.
- Hooper, D. J., Wilson, M. J., Rowe, J. A., & Glen, D. M. (1999). Some observations on the morphology and protein profiles of the slug-parasitic nematodes Phasmarhabditis hermaphrodita and P. neopapillosa (Nematoda: Rhabditidae). Nematology, 1(2), 173-182.
- De Ley, I. T., Holovachov, O., Mc Donnell, R. J., Bert, W., Paine, T. D., & De Ley, P. (2016). Description of Phasmarhabditis californica n. sp. and first report of P. papillosa (Nematoda: Rhabditidae) from invasive slugs in the USA. Nematology, 18(2), 175-193.
- Nermuť, J., Půža, V., & Mráček, Z. (2016). Phasmarhabditis apuliae n. sp.(Nematoda: Rhabditidae), a new rhabditid nematode from milacid slugs.
- Nermuť, J., Půža, V., Mekete, T., & Mráček, Z. (2016). Phasmarhabditis bohemica n. sp. (Nematoda: Rhabditidae), a slug-parasitic nematode from the Czech Republic.
- Nermuť, J., Půža, V., Mekete, T., & Mráček, Z. (2016). Phasmarhabditis bonaquaense n. sp. (Nematoda: Rhabditidae), a new slug-parasitic nematode from the Czech Republic. Zootaxa, 4179(3), 530-531.
- Huang, R. E., Ye, W., Ren, X., & Zhao, Z. (2015). Morphological and Molecular Characterization of Phasmarhabditis huizhouensis sp. nov. (Nematoda: Rhabditidae), a New Rhabditid Nematode from South China. PLoS ONE, 10(12).
- Azzam, K. M. (2003). Description of the nematode Phasmarhabditis tawfiki n. sp. isolated from Egyptian terrestrial snails and slugs. JOURNAL-EGYPTIAN GERMAN SOCIETY OF ZOOLOGY, 42(D), 79-88.
- Wilson, M. J., Glen, D. M., & George, S. K. (1993). The rhabditid nematode Phasmarhabditis hermaphrodita as a potential biological control agent for slugs. Biocontrol Science and Technology, 3(4), 503-511.
- Rae, R., Verdun, C., Grewal, P. S., Robertson, J. F., & Wilson, M. J. (2007). Biological control of terrestrial molluscs using Phasmarhabditis hermaphrodita—progress and prospects. Pest Management Science, 63(12), 1153-1164.
- Lee, D. L. (Ed.). (2002). The biology of nematodes. CRC Press. 191-191; 294-295
- Wilson, M. J., Glen, D. M., George, S. K., & Butler, R. C. (1993). Mass cultivation and storage of the rhabditid nematode Phasmarhabditis hermaphrodita, a biocontrol agent for slugs. Biocontrol Science and Technology, 3(4), 513-521.
- Maupas, E, (1900) Modes et formes de reproduction des nématodes. Archives de Zoologie Expérimentale et Générale 7, 563-628.
- Riddle, D. L., & Albert, P. S. (1997). 26 Genetic and Environmental Regulation of Dauer Larva Development. Cold Spring Harbor Monograph Archive, 33, 739-768.
- Ludewig, A. H., Kober-Eisermann, C., Weitzel, C., Bethke, A., Neubert, K., Gerisch, B., ... & Antebi, A. (2004). A novel nuclear receptor/coregulator complex controls C. elegans lipid metabolism, larval development, and aging. Genes & development, 18(17), 2120-2133.
- Tan, L., & Grewal, P. S. (2001a). Infection behavior of the rhabditid nematode Phasmarhabditis hermaphrodita to the grey garden slug Deroceras reticulatum. Journal of Parasitology, 87(6), 1349-1354.
- Tan, L., & Grewal, P. S. (2001b). Pathogenicity of Moraxella osloensis, a bacterium associated with the nematode Phasmarhabditis hermaphrodita, to the slug Deroceras reticulatum. Applied and Environmental Microbiology, 67(11), 5010-5016.
- Wilson, M., & Rae, R. (2015). Phasmarhabditis hermaphrodita as a Control Agent for Slugs. In Nematode Pathogenesis of Insects and Other Pests. 509-521. Springer International Publishing.
- Williams, A. J., & Rae, R. (2015). Susceptibility of the Giant African Snail (Achatina fulica) exposed to the gastropod parasitic nematode Phasmarhabditis hermaphrodita. Journal of invertebrate pathology, 127, 122-126.
- Nicholls, C. (2014) Implications of not controlling slugs in oilseed rape and wheat in the UK. Research Review No. 79. [PDF] Kenilworth: Agricultural & Horticulture Development Board. 4-5.
- Grewal, S. K., Grewal, P. S., & Hammond, R. B. (2003). Susceptibility of North American native and non-native slugs (Mollusca: Gastropoda) to Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae). Biocontrol Science and Technology, 13(1), 119-125.
- Pieterse, A., Malan, A. P., & Ross, J. L. (2016). Nematodes that associate with terrestrial molluscs as definitive hosts, including Phasmarhabditis hermaphrodita (Rhabditida: Rhabditidae) and its development as a biological molluscicide. Journal of Helminthology, 1-11.
- Iglesias, J., Castillejo, J., & Castro, R. (2003). The effects of repeated applications of the molluscicide metaldehyde and the biocontrol nematode Phasmarhabditis hermaphrodita on molluscs, earthworms, nematodes, acarids and collembolans: a two‐year study in north‐west Spain. Pest management science, 59(11), 1217-1224.
- Wilson, M. J., Glen, D. M., George, S. K., Pearce, J. D., & Wiltshire, C. W. (1994). Biological control of slugs in winter wheat using the rhabditid nematode Phasmarhabditis hermaphrodita. Annals of Applied Biology, 125(2), 377-390.
- Wilson, M. J., Glen, D. M., George, S. K., & Hughes, L. A. (1995). Biocontrol of slugs in protected lettuce using the rhabditid nematode Phasmarhabditis hermaphrodita. Biocontrol Science and technology, 5(2), 233-242
- Wilson, M. J., Hughes, L. A., Hamacher, G. M., Barahona, L. D., & Glen, D. M. (1996). Effects of soil incorporation on the efficacy of the rhabditid nematode, Phasmarhabditis hermaphrodita, as a biological control agent for slugs. Annals of applied biology, 128(1), 117-126.
- Lacey, L. A., & Kaya, H. K. (2007). Field manual of techniques in invertebrate pathology. Dordrecht etc.: Kluwer acad. publ. Cop. 2000. 789–790.
- Wilson, M. J., Hughes, L. A., Jefferies, D., & Glen, D. M. (1999). Slugs (Deroceras reticulatum and Arion ater agg.) avoid soil treated with the rhabditid nematode Phasmarhabditis hermaphrodita. Biological Control, 16(2), 170-176.
- Hapca, S., Crawford, J., Rae, R., Wilson, M., & Young, I. (2007). Movement of the parasitic nematode Phasmarhabditis hermaphrodita in the presence of mucus from the host slug Deroceras reticulatum. Biological Control, 41(2), 223-229.
- Rae, R. G., Tourna, M., & Wilson, M. J. (2010). The slug parasitic nematode Phasmarhabditis hermaphrodita associates with complex and variable bacterial assemblages that do not affect its virulence. Journal of invertebrate pathology, 104(3), 222-226.
- Pechova, H., & Foltan, P. (2008). The parasitic nematode Phasmarhabditis hermaphrodita defends its slug host from being predated or scavenged by manipulating host spatial behaviour. Behavioural processes, 78(3), 416-420.
- Foltan, P., & Puza, V. (2009). To complete their life cycle, pathogenic nematode–bacteria complexes deter scavengers from feeding on their host cadaver. Behavioural processes, 80(1), 76-79.
- Rae, R. G., Tourna, M., & Wilson, M. J. (2010). The slug parasitic nematode Phasmarhabditis hermaphrodita associates with complex and variable bacterial assemblages that do not affect its virulence. Journal of invertebrate pathology, 104(3), 225-226.
Further reading
- Coupland, J. B. (1995). "Susceptibility of Helicid snails to isolates of the nematode Phasmarhabditis hermaphrodita from Southern France". Journal of Invertebrate Pathology. 66: 207–208. doi:10.1006/jipa.1995.1088.
- Grimm, B. (2002). "Effect of the nematode Phasmarhabditis hermaphrodita on young stages of the pest slug Arion lusitanicus". Journal of Molluscan Studies. 68: 25–28. doi:10.1093/mollus/68.1.25.