Octopus
The octopus (plural octopuses) is a soft-bodied, eight-limbed mollusc of the order Octopoda (/ɒkˈtɒpədə/, ok-TO-pə-də). Around 300 species are recognised, and the order is grouped within the class Cephalopoda with squids, cuttlefish, and nautiloids. Like other cephalopods, the octopus is bilaterally symmetric with two eyes and a beak, with its mouth at the center point of the eight limbs ("tentacle" is used as an umbrella term for cephalopod limbs; however, within a teuthological context, "arm" is used to refer to such limbs while "tentacle" is reserved for feeding appendages not found on octopuses). The soft body can rapidly alter its shape, enabling octopuses to squeeze through small gaps. They trail their eight appendages behind them as they swim. The siphon is used both for respiration and for locomotion, by expelling a jet of water. Octopuses have a complex nervous system and excellent sight, and are among the most intelligent and behaviourally diverse of all invertebrates.
| Octopus | |
|---|---|
 | |
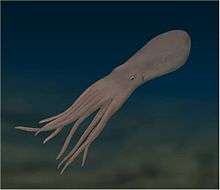
| Common octopus (Octopus vulgaris) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Mollusca |
| Class: | Cephalopoda |
| Subclass: | Coleoidea |
| (unranked): | Neocoleoidea |
| Superorder: | Octopodiformes |
| Order: | Octopoda Leach, 1818[1] |
| Suborders | |
|
(traditional) See § Evolution for families | |
| Synonyms | |
| |
Octopuses inhabit various regions of the ocean, including coral reefs, pelagic waters, and the seabed; some live in the intertidal zone and others at abyssal depths. Most species grow quickly, mature early, and are short-lived. In most species, the male uses a specially adapted arm to deliver a bundle of sperm directly into the female's mantle cavity, after which he becomes senescent and dies, while the female deposits fertilised eggs in a den and cares for them until they hatch, after which she also dies. Strategies to defend themselves against predators include the expulsion of ink, the use of camouflage and threat displays, the ability to jet quickly through the water and hide, and even deceit. All octopuses are venomous, but only the blue-ringed octopuses are known to be deadly to humans.
Octopuses appear in mythology as sea monsters like the Kraken of Norway and the Akkorokamui of the Ainu, and probably the Gorgon of ancient Greece. A battle with an octopus appears in Victor Hugo's book Toilers of the Sea, inspiring other works such as Ian Fleming's Octopussy. Octopuses appear in Japanese erotic art, shunga. They are eaten and considered a delicacy by humans in many parts of the world, especially the Mediterranean and the Asian seas.
Etymology and pluralisation
The scientific Latin term octopus was derived from Ancient Greek ὀκτώπους, a compound form of ὀκτώ (oktō, "eight") and πούς (pous, "foot"), itself a variant form of ὀκτάπους, a word used for example by Alexander of Tralles (c. 525–605) for the common octopus.[3][4][5] The standard pluralised form of "octopus" in English is "octopuses";[6] the Ancient Greek plural ὀκτώποδες, "octopodes" (/ɒkˈtɒpədiːz/), has also been used historically.[7] The alternative plural "octopi" is considered grammatically incorrect because it wrongly assumes that octopus is a Latin second declension "-us" noun or adjective when, in either Greek or Latin, it is a third declension noun.[8][9] Fowler's Modern English Usage states that the only acceptable plural in English is "octopuses", that "octopi" is misconceived, and "octopodes" pedantic;[10][11][12] the latter is nonetheless used frequently enough to be acknowledged by the descriptivist Merriam-Webster 11th Collegiate Dictionary and Webster's New World College Dictionary. The Oxford English Dictionary lists "octopuses", "octopi", and "octopodes", in that order, reflecting frequency of use, calling "octopodes" rare and noting that "octopi" is based on a misunderstanding.[13] The New Oxford American Dictionary (3rd Edition, 2010) lists "octopuses" as the only acceptable pluralisation, and indicates that "octopodes" is still occasionally used, but that "octopi" is incorrect.[14]
Anatomy and physiology
Size
The giant Pacific octopus (Enteroctopus dofleini) is often cited as the largest known octopus species. Adults usually weigh around 15 kg (33 lb), with an arm span of up to 4.3 m (14 ft).[15] The largest specimen of this species to be scientifically documented was an animal with a live mass of 71 kg (156.5 lb).[16] Much larger sizes have been claimed for the giant Pacific octopus:[17] one specimen was recorded as 272 kg (600 lb) with an arm span of 9 m (30 ft).[18] A carcass of the seven-arm octopus, Haliphron atlanticus, weighed 61 kg (134 lb) and was estimated to have had a live mass of 75 kg (165 lb).[19][20] The smallest species is Octopus wolfi, which is around 2.5 cm (1 in) and weighs less than 1 g (0.035 oz).[21]
External characteristics
The octopus is bilaterally symmetrical along its dorso-ventral axis; the head and foot are at one end of an elongated body and function as the anterior (front) of the animal. The head includes the mouth and brain. The foot has evolved into a set of flexible, prehensile appendages, known as "arms", that surround the mouth and are attached to each other near their base by a webbed structure.[22] The arms can be described based on side and sequence position (such as L1, R1, L2, R2) and divided into four pairs.[23][22] The two rear appendages are generally used to walk on the sea floor, while the other six are used to forage for food; hence some biologists refer to the animals as having six "arms" and two "legs".[24][25] The bulbous and hollow mantle is fused to the back of the head and is known as the visceral hump; it contains most of the vital organs.[26][27] The mantle cavity has muscular walls and contains the gills; it is connected to the exterior by a funnel or siphon.[22][28] The mouth of an octopus, located underneath the arms, has a sharp hard beak.[27]
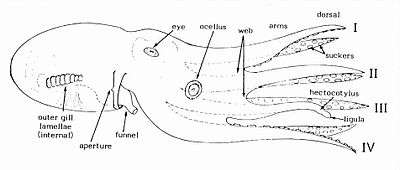
The skin consists of a thin outer epidermis with mucous cells and sensory cells, and a connective tissue dermis consisting largely of collagen fibres and various cells allowing colour change.[22] Most of the body is made of soft tissue allowing it to lengthen, contract, and contort itself. The octopus can squeeze through tiny gaps; even the larger species can pass through an opening close to 2.5 cm (1 in) in diameter.[27] Lacking skeletal support, the arms work as muscular hydrostats and contain longitudinal, transverse and circular muscles around a central axial nerve. They can extend and contract, twist to left or right, bend at any place in any direction or be held rigid.[29][30]
The interior surfaces of the arms are covered with circular, adhesive suckers. The suckers allow the octopus to anchor itself or to manipulate objects. Each sucker is usually circular and bowl-like and has two distinct parts: an outer shallow cavity called an infundibulum and a central hollow cavity called an acetabulum, both of which are thick muscles covered in a protective chitinous cuticle. When a sucker attaches to a surface, the orifice between the two structures is sealed. The infundibulum provides adhesion while the acetabulum remains free, and muscle contractions allow for attachment and detachment.[31][32]
.jpg)
The eyes of the octopus are large and are at the top of the head. They are similar in structure to those of a fish and are enclosed in a cartilaginous capsule fused to the cranium. The cornea is formed from a translucent epidermal layer and the slit-shaped pupil forms a hole in the iris and lies just behind. The lens is suspended behind the pupil and photoreceptive retinal cells cover the back of the eye. The pupil can be adjusted in size and a retinal pigment screens incident light in bright conditions.[22]
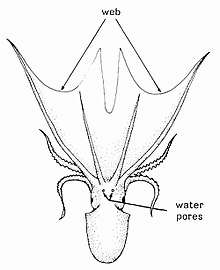
Some species differ in form from the typical octopus body shape. Basal species, the Cirrina, have stout gelatinous bodies with webbing that reaches near the tip of their arms, and two large fins above the eyes, supported by an internal shell. Fleshy papillae or cirri are found along the bottom of the arms, and the eyes are more developed.[33][34]
Circulatory system
Octopuses have a closed circulatory system, in which the blood remains inside blood vessels. Octopuses have three hearts; a systemic heart that circulates blood around the body and two branchial hearts that pump it through each of the two gills. The systemic heart is inactive when the animal is swimming and thus it tires quickly and prefers to crawl.[35][36] Octopus blood contains the copper-rich protein haemocyanin to transport oxygen. This makes the blood very viscous and it requires considerable pressure to pump it around the body; octopuses' blood pressures can exceed 75 mmHg (10 kPa).[35][36][37] In cold conditions with low oxygen levels, haemocyanin transports oxygen more efficiently than haemoglobin. The haemocyanin is dissolved in the plasma instead of being carried within blood cells, and gives the blood a bluish colour.[35][36]
The systemic heart has muscular contractile walls and consists of a single ventricle and two atria, one for each side of the body. The blood vessels consist of arteries, capillaries and veins and are lined with a cellular endothelium which is quite unlike that of most other invertebrates. The blood circulates through the aorta and capillary system, to the vena cavae, after which the blood is pumped through the gills by the auxiliary hearts and back to the main heart. Much of the venous system is contractile, which helps circulate the blood.[22]
Respiration

Respiration involves drawing water into the mantle cavity through an aperture, passing it through the gills, and expelling it through the siphon. The ingress of water is achieved by contraction of radial muscles in the mantle wall, and flapper valves shut when strong circular muscles force the water out through the siphon.[38] Extensive connective tissue lattices support the respiratory muscles and allow them to expand the respiratory chamber.[39] The lamella structure of the gills allows for a high oxygen uptake, up to 65% in water at 20 °C (68 °F).[40] Water flow over the gills correlates with locomotion, and an octopus can propel its body when it expels water out of its siphon.[39][37]
The thin skin of the octopus absorbs additional oxygen. When resting, around 41% of an octopus's oxygen absorption is through the skin. This decreases to 33% when it swims, as more water flows over the gills; skin oxygen uptake also increases. When it is resting after a meal, absorption through the skin can drop to 3% of its total oxygen uptake.[41]
Digestion and excretion
The digestive system of the octopus begins with the buccal mass which consists of the mouth with its chitinous beak, the pharynx, radula and salivary glands.[42] The radula is a spiked, muscular tongue-like organ with multiple rows of tiny teeth.[27] Food is broken down and is forced into the oesophagus by two lateral extensions of the esophageal side walls in addition to the radula. From there it is transferred to the gastrointestinal tract, which is mostly suspended from the roof of the mantle cavity by numerous membranes. The tract consists of a crop, where the food is stored; a stomach, where food is ground down; a caecum where the now sludgy food is sorted into fluids and particles and which plays an important role in absorption; the digestive gland, where liver cells break down and absorb the fluid and become "brown bodies"; and the intestine, where the accumulated waste is turned into faecal ropes by secretions and blown out of the funnel via the rectum.[42][43]
During osmoregulation, fluid is added to the pericardia of the branchial hearts. The octopus has two nephridia (equivalent to vertebrate kidneys) which are associated with the branchial hearts; these and their associated ducts connect the pericardial cavities with the mantle cavity. Before reaching the branchial heart, each branch of the vena cava expands to form renal appendages which are in direct contact with the thin-walled nephridium. The urine is first formed in the pericardial cavity, and is modified by excretion, chiefly of ammonia, and selective absorption from the renal appendages, as it is passed along the associated duct and through the nephridiopore into the mantle cavity.[22][44]
Nervous system and senses
The octopus (along with cuttlefish) has the highest brain-to-body mass ratios of all invertebrates; it is also greater than that of many vertebrates.[45][46] It has a highly complex nervous system, only part of which is localised in its brain, which is contained in a cartilaginous capsule.[47] Two-thirds of an octopus's neurons are found in the nerve cords of its arms, which show a variety of complex reflex actions that persist even when they have no input from the brain.[48] Unlike vertebrates, the complex motor skills of octopuses are not organised in their brain via an internal somatotopic map of its body, instead using a nonsomatotopic system unique to large-brained invertebrates.[49]
Like other cephalopods, octopuses can distinguish the polarisation of light. Colour vision appears to vary from species to species, for example being present in O. aegina but absent in O. vulgaris.[50] Researchers believe that opsins in the skin can sense different wavelengths of light and help the creatures choose a coloration that camouflages them, in addition to light input from the eyes.[51] Other researchers hypothesise that cephalopod eyes in species which only have a single photoreceptor protein may use chromatic aberration to turn monochromatic vision into colour vision, though this sacrifices image quality.[52] This would explain pupils shaped like the letter U, the letter W, or a dumbbell, as well as explaining the need for colourful mating displays.[53]
Attached to the brain are two special organs called statocysts (sac-like structures containing a mineralised mass and sensitive hairs), that allow the octopus to sense the orientation of its body. They provide information on the position of the body relative to gravity and can detect angular acceleration. An autonomic response keeps the octopus's eyes oriented so that the pupil is always horizontal.[22] Octopuses may also use the statocyst to hear sound. The common octopus can hear sounds between 400 Hz and 1000 Hz, and hears best at 600 Hz.[54]
Octopuses also have an excellent sense of touch. The octopus's suction cups are equipped with chemoreceptors so the octopus can taste what it touches. Octopus arms do not become tangled or stuck to each other because the sensors recognise octopus skin and prevent self-attachment.[55]
The arms contain tension sensors so the octopus knows whether its arms are stretched out, but this is not sufficient for the brain to determine the position of the octopus's body or arms. As a result, the octopus does not possess stereognosis; that is, it does not form a mental image of the overall shape of the object it is handling. It can detect local texture variations, but cannot integrate the information into a larger picture. The neurological autonomy of the arms means the octopus has great difficulty learning about the detailed effects of its motions. It has a poor proprioceptive sense, and it knows what exact motions were made only by observing the arms visually.[56]
Ink sac
The ink sac of an octopus is located under the digestive gland. A gland attached to the sac produces the ink, and the sac stores it. The sac is close enough to the funnel for the octopus to shoot out the ink with a water jet. Before it leaves the funnel, the ink passes through glands which mix it with mucus, creating a thick, dark blob which allows the animal to escape from a predator.[57] The main pigment in the ink is melanin, which gives it its black colour.[58] Cirrate octopuses lack the ink sac.[33]
Lifecycle
Reproduction
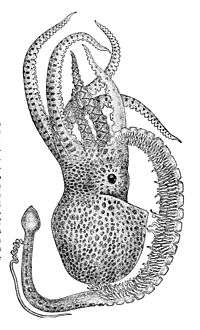
Octopuses are gonochoric and have a single, posteriorly-located gonad which is associated with the coelom. The testis in males and the ovary in females bulges into the gonocoel and the gametes are released here. The gonocoel is connected by the gonoduct to the mantle cavity, which it enters at the gonopore.[22] An optic gland creates hormones that cause the octopus to mature and age and stimulate gamete production. The gland may be triggered by environmental conditions such as temperature, light and nutrition, which thus control the timing of reproduction and lifespan.[59][60]
When octopuses reproduce, the male uses a specialised arm called a hectocotylus to transfer spermatophores (packets of sperm) from the terminal organ of the reproductive tract (the cephalopod "penis") into the female's mantle cavity.[61] The hectocotylus in benthic octopuses is usually the third right arm, which has a spoon-shaped depression and modified suckers near the tip. In most species, fertilisation occurs in the mantle cavity.[22]
The reproduction of octopuses has been studied in only a few species. One such species is the giant Pacific octopus, in which courtship is accompanied, especially in the male, by changes in skin texture and colour. The male may cling to the top or side of the female or position himself beside her. There is some speculation that he may first use his hectocotylus to remove any spermatophore or sperm already present in the female. He picks up a spermatophore from his spermatophoric sac with the hectocotylus, inserts it into the female's mantle cavity, and deposits it in the correct location for the species, which in the giant Pacific octopus is the opening of the oviduct. Two spermatophores are transferred in this way; these are about one metre (yard) long, and the empty ends may protrude from the female's mantle.[62] A complex hydraulic mechanism releases the sperm from the spermatophore, and it is stored internally by the female.[22]

About forty days after mating, the female giant Pacific octopus attaches strings of small fertilised eggs (10,000 to 70,000 in total) to rocks in a crevice or under an overhang. Here she guards and cares for them for about five months (160 days) until they hatch.[62] In colder waters, such as those off of Alaska, it may take as much as 10 months for the eggs to completely develop.[63]:74 The female aerates the eggs and keeps them clean; if left untended, many eggs will not hatch.[64] She does not feed during this time and dies soon afterwards. Males become senescent and die a few weeks after mating.[65]
The eggs have large yolks; cleavage (division) is superficial and a germinal disc develops at the pole. During gastrulation, the margins of this grow down and surround the yolk, forming a yolk sac, which eventually forms part of the gut. The dorsal side of the disc grows upwards and forms the embryo, with a shell gland on its dorsal surface, gills, mantle and eyes. The arms and funnel develop as part of the foot on the ventral side of the disc. The arms later migrate upwards, coming to form a ring around the funnel and mouth. The yolk is gradually absorbed as the embryo develops.[22]

Most young octopuses hatch as paralarvae and are planktonic for weeks to months, depending on the species and water temperature. They feed on copepods, arthropod larvae and other zooplankton, eventually settling on the ocean floor and developing directly into adults with no distinct metamorphoses that are present in other groups of mollusc larvae.[22] Octopus species that produce larger eggs – including the southern blue-ringed, Caribbean reef, California two-spot, Eledone moschata[66] and deep sea octopuses – do not have a paralarval stage, but hatch as benthic animals similar to the adults.[63]:74–75[67]
In the argonaut (paper nautilus), the female secretes a fine, fluted, papery shell in which the eggs are deposited and in which she also resides while floating in mid-ocean. In this she broods the young, and it also serves as a buoyancy aid allowing her to adjust her depth. The male argonaut is minute by comparison and has no shell.[68]
Lifespan
Octopuses have a relatively short life expectancy; some species live for as little as six months. The giant Pacific octopus, one of the two largest species of octopus, may live for as much as five years. Octopus lifespan is limited by reproduction: males can live for only a few months after mating, and females die shortly after their eggs hatch. The larger Pacific striped octopus is an exception, as it can reproduce multiple times over a life of around two years.[69] Octopus reproductive organs mature due to the hormonal influence of the optic gland but result in the inactivation of their digestive glands, typically causing the octopus to die from starvation.[70]:276–277 Experimental removal of both optic glands after spawning was found to result in the cessation of broodiness, the resumption of feeding, increased growth, and greatly extended lifespans.[71]
Distribution and habitat

Octopuses live in every ocean, and different species have adapted to different marine habitats. As juveniles, common octopuses inhabit shallow tide pools. The Hawaiian day octopus (Octopus cyanea) lives on coral reefs; argonauts drift in pelagic waters. Abdopus aculeatus mostly lives in near-shore seagrass beds. Some species are adapted to the cold, ocean depths. The spoon-armed octopus (Bathypolypus arcticus) is found at depths of 1,000 m (3,300 ft), and Vulcanoctopus hydrothermalis lives near hydrothermal vents at 2,000 m (6,600 ft).[26] The cirrate species are often free-swimming and live in deep-water habitats.[34] Although several species are known to live at bathyal and abyssal depths, there is only a single indisputable record of an octopus in the hadal zone; a species of Grimpoteuthis (dumbo octopus) photographed at 6,957 m (22,825 ft).[72] No species are known to live in fresh water.[73]
Behaviour and ecology
Most species are solitary when not mating,[74] though a few are known to occur in high densities and with frequent interactions, signaling, mate defending and eviction of individuals from dens. This is likely the result of abundant food supplies combined with limited den sites.[75] The larger Pacific striped octopus however is social, living in groups of up to 40 individuals that share dens.[69] Octopuses hide in dens, which are typically crevices in rocky outcrops or other hard structures, though some species burrow into sand or mud. Octopuses are not territorial but generally remain in a home range; they may leave the area in search of food. They can use navigation skills to return to a den without having to retrace their outward route.[76] They are not known to be migratory.[77]
Octopuses bring captured prey back to the den where they can eat it safely. Sometimes the octopus catches more prey than it can eat, and the den is often surrounded by a midden of dead and uneaten food items. Other creatures, such as fish, crabs, molluscs and echinoderms, often share the den with the octopus, either because they have arrived as scavengers, or because they have survived capture.[78]
Feeding

Nearly all octopuses are predatory; bottom-dwelling octopuses eat mainly crustaceans, polychaete worms, and other molluscs such as whelks and clams; open-ocean octopuses eat mainly prawns, fish and other cephalopods.[79] Major items in the diet of the giant Pacific octopus include bivalve molluscs such as the cockle Clinocardium nuttallii, clams and scallops and crustaceans such as crabs and spider crabs. Prey that it is likely to reject include moon snails because they are too large and limpets, rock scallops, chitons and abalone, because they are too securely fixed to the rock.[78]
A benthic (bottom-dwelling) octopus typically moves among the rocks and feels through the crevices. The creature may make a jet-propelled pounce on prey and pull it towards the mouth with its arms, the suckers restraining it. Small prey may be completely trapped by the webbed structure. Octopuses usually inject crustaceans like crabs with a paralysing saliva then dismember them with their beaks.[79][80] Octopuses feed on shelled molluscs either by forcing the valves apart, or by drilling a hole in the shell to inject a nerve toxin.[81][80] It used to be thought that the hole was drilled by the radula, but it has now been shown that minute teeth at the tip of the salivary papilla are involved, and an enzyme in the toxic saliva is used to dissolve the calcium carbonate of the shell. It takes about three hours for O. vulgaris to create a 0.6 mm (0.024 in) hole. Once the shell is penetrated, the prey dies almost instantaneously, its muscles relax, and the soft tissues are easy for the octopus to remove. Crabs may also be treated in this way; tough-shelled species are more likely to be drilled, and soft-shelled crabs are torn apart.[82]
Some species have other modes of feeding. Grimpoteuthis has a reduced or non-existent radula and swallows prey whole.[33] In the deep-sea genus Stauroteuthis, some of the muscle cells that control the suckers in most species have been replaced with photophores which are believed to fool prey by directing them towards the mouth, making them one of the few bioluminescent octopuses.[83]
Locomotion

Octopuses mainly move about by relatively slow crawling with some swimming in a head-first position. Jet propulsion or backwards swimming, is their fastest means of locomotion, followed by swimming and crawling.[84] When in no hurry, they usually crawl on either solid or soft surfaces. Several arms are extended forwards, some of the suckers adhere to the substrate and the animal hauls itself forwards with its powerful arm muscles, while other arms may push rather than pull. As progress is made, other arms move ahead to repeat these actions and the original suckers detach. During crawling, the heart rate nearly doubles, and the animal requires ten or fifteen minutes to recover from relatively minor exercise.[29]
Most octopuses swim by expelling a jet of water from the mantle through the siphon into the sea. The physical principle behind this is that the force required to accelerate the water through the orifice produces a reaction that propels the octopus in the opposite direction.[85] The direction of travel depends on the orientation of the siphon. When swimming, the head is at the front and the siphon is pointed backwards, but when jetting, the visceral hump leads, the siphon points towards the head and the arms trail behind, with the animal presenting a fusiform appearance. In an alternative method of swimming, some species flatten themselves dorso-ventrally, and swim with the arms held out sideways, and this may provide lift and be faster than normal swimming. Jetting is used to escape from danger, but is physiologically inefficient, requiring a mantle pressure so high as to stop the heart from beating, resulting in a progressive oxygen deficit.[84]
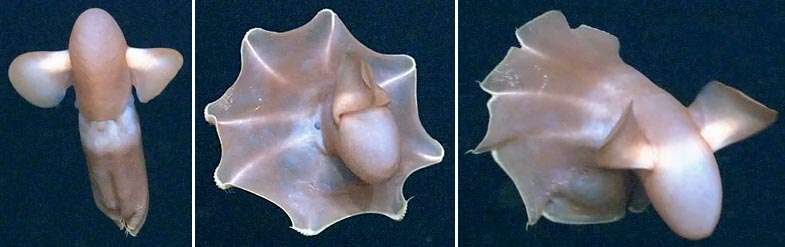
Cirrate octopuses cannot produce jet propulsion and rely on their fins for swimming. They have neutral buoyancy and drift through the water with the fins extended. They can also contract their arms and surrounding web to make sudden moves known as "take-offs". Another form of locomotion is "pumping", which involves symmetrical contractions of muscles in their webs producing peristaltic waves. This moves the body slowly.[33]
In 2005, Adopus aculeatus and veined octopus (Amphioctopus marginatus) were found to walk on two arms, while at the same time mimicking plant matter.[86] This form of locomotion allows these octopuses to move quickly away from a potential predator without being recognised.[84] A study of this behaviour led to the suggestion that the two rearmost appendages may be more accurately termed "legs" rather than "arms".[87] Some species of octopus can crawl out of the water briefly, which they may do between tide pools while hunting crustaceans or gastropods or to escape predators.[88][89] "Stilt walking" is used by the veined octopus when carrying stacked coconut shells. The octopus carries the shells underneath it with two arms, and progresses with an ungainly gait supported by its remaining arms held rigid.[90]
Intelligence
.jpg)
Octopuses are highly intelligent; the extent of their intelligence and learning capability are not well defined.[91][92][93][94] Maze and problem-solving experiments have shown evidence of a memory system that can store both short- and long-term memory. It is not known precisely what contribution learning makes to adult octopus behaviour. Young octopuses learn nothing from their parents, as adults provide no parental care beyond tending to their eggs until the young octopuses hatch.[63]:75
In laboratory experiments, octopuses can be readily trained to distinguish between different shapes and patterns. They have been reported to practise observational learning,[95] although the validity of these findings is contested.[91][92] Octopuses have also been observed in what has been described as play: repeatedly releasing bottles or toys into a circular current in their aquariums and then catching them.[96] Octopuses often break out of their aquariums and sometimes into others in search of food.[88][97][98] They have even boarded fishing boats and opened holds to eat crabs.[93] The veined octopus collects discarded coconut shells, then uses them to build a shelter, an example of tool use.[90][99][100]
Camouflage and colour change
Octopuses use camouflage when hunting and to avoid predators. To do this they use specialised skin cells which change the appearance of the skin by adjusting its colour, opacity, or reflectivity. Chromatophores contain yellow, orange, red, brown, or black pigments; most species have three of these colours, while some have two or four. Other colour-changing cells are reflective iridophores and white leucophores.[101] This colour-changing ability is also used to communicate with or warn other octopuses.[102]
Octopuses can create distracting patterns with waves of dark coloration across the body, a display known as the "passing cloud". Muscles in the skin change the texture of the mantle to achieve greater camouflage. In some species, the mantle can take on the spiky appearance of algae; in others, skin anatomy is limited to relatively uniform shades of one colour with limited skin texture. Octopuses that are diurnal and live in shallow water have evolved more complex skin than their nocturnal and deep-sea counterparts.[102]
A "moving rock" trick involves the octopus mimicking a rock and then inching across the open space with a speed matching the movement in the surrounding water, allowing it to move in plain sight of a predator.[94][103]
Defence
Aside from humans, octopuses may be preyed on by fishes, seabirds, sea otters, pinnipeds, cetaceans, and other cephalopods.[104] Octopuses typically hide or disguise themselves by camouflage and mimicry; some have conspicuous warning coloration (aposematism) or deimatic behaviour.[102] An octopus may spend 40% of its time hidden away in its den. When the octopus is approached, it may extend an arm to investigate. 66% of Enteroctopus dofleini in one study had scars, with 50% having amputated arms.[104] The blue rings of the highly venomous blue-ringed octopus are hidden in muscular skin folds which contract when the animal is threatened, exposing the iridescent warning.[105] The Atlantic white-spotted octopus (Callistoctopus macropus) turns bright brownish red with oval white spots all over in a high contrast display.[106] Displays are often reinforced by stretching out the animal's arms, fins or web to make it look as big and threatening as possible.[107]
Once they have been seen by a predator, they commonly try to escape but can also use distraction with an ink cloud ejected from the ink sac. The ink is thought to reduce the efficiency of olfactory organs, which would aid evasion from predators that employ smell for hunting, such as sharks. Ink clouds of some species might act as pseudomorphs, or decoys that the predator attacks instead.[108]
When under attack, some octopuses can perform arm autotomy, in a manner similar to the way skinks and other lizards detach their tails. The crawling arm may distract would-be predators. Such severed arms remain sensitive to stimuli and move away from unpleasant sensations.[109] Octopuses can replace lost limbs.[110]
Some octopuses, such as the mimic octopus, can combine their highly flexible bodies with their colour-changing ability to mimic other, more dangerous animals, such as lionfish, sea snakes, and eels.[111][112]
Pathogens and parasites
The diseases and parasites that affect octopuses have been little studied, but cephalopods are known to be the intermediate or final hosts of various parasitic cestodes, nematodes and copepods; 150 species of protistan and metazoan parasites have been recognised.[113] The Dicyemidae are a family of tiny worms that are found in the renal appendages of many species;[114] it is unclear whether they are parasitic or are endosymbionts. Coccidians in the genus Aggregata living in the gut cause severe disease to the host. Octopuses have an innate immune system, and the haemocytes respond to infection by phagocytosis, encapsulation, infiltration or cytotoxic activities to destroy or isolate the pathogens. The haemocytes play an important role in the recognition and elimination of foreign bodies and wound repair. Captive animals have been found to be more susceptible to pathogens than wild ones.[115] A gram-negative bacterium, Vibrio lentus, has been found to cause skin lesions, exposure of muscle and death of octopuses in extreme cases.[116]
Evolution
The scientific name Octopoda was first coined and given as the order of octopuses in 1818 by English biologist William Elford Leach,[117] who classified them as Octopoida the previous year.[2] The Octopoda consists of around 300 known species[118] and were historically divided into two suborders, the Incirrina and the Cirrina.[34] However, more recent evidence suggests that Cirrina are merely the most basal species and are not a unique clade.[119] The incirrate octopuses (the majority of species) lack the cirri and paired swimming fins of the cirrates.[34] In addition, the internal shell of incirrates is either present as a pair of stylets or absent altogether.[120]
- Order Octopoda
 Life restoration of Keuppia levante, an extinct species from the Cretaceous
Life restoration of Keuppia levante, an extinct species from the Cretaceous- Genus †Keuppia (incertae sedis)
- Genus †Palaeoctopus (incertae sedis)
- Genus †Paleocirroteuthis (incertae sedis)
- Genus †Pohlsepia (incertae sedis)
- Genus †Proteroctopus (incertae sedis)
- Genus †Styletoctopus (incertae sedis)
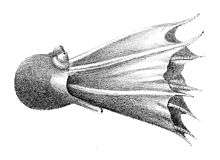
- Suborder Cirrina: finned deep-sea octopus
- Family Opisthoteuthidae: umbrella octopus
- Family Cirroctopodidae
- Family Cirroteuthidae
- Family Stauroteuthidae
- Suborder Incirrina
- Superfamily Octopodoidea[121]
- Family Amphitretidae
- Family Bathypolypodidae
- Family Eledonidae
- Family Enteroctopodidae
- Family Megaleledonidae Taki, 1961
- Family Octopodidae
- Superfamily Argonautoidea
- Family Alloposidae: seven-arm octopus
- Family Argonautidae: argonauts
- Family Ocythoidae: tuberculate pelagic octopus
- Family Tremoctopodidae: blanket octopus
- Superfamily Octopodoidea[121]
Fossil history and phylogeny
Cephalopods have existed for 500 million years and octopus ancestors were in the Carboniferous seas 300 million years ago. The oldest known octopus fossil is Pohlsepia, which lived 296 million years ago. Researchers have identified impressions of eight arms, two eyes, and possibly an ink sac.[122] Octopuses are mostly soft tissue, and so fossils are relatively rare. Octopuses, squids and cuttlefish belong to the clade Coleoidea. They are known as "soft-bodied" cephalopods, lacking the external shell of most molluscs and other cephalopods like the nautiloids and the extinct Ammonoidea.[123] Octopuses have eight limbs like other coleoids but lack the extra specialised feeding appendages known as tentacles which are longer and thinner with suckers only at their club-like ends.[124][125][126] The vampire squid (Vampyroteuthis) also lacks tentacles but has sensory filaments.[127]
The cladograms are based on Sanchez et al., 2018, who created a molecular phylogeny based on mitochondrial and nuclear DNA marker sequences.[119]
| Cephalopods |
| ||||||||||||||||||
The molecular analysis of the octopods shows that the suborder Cirrina (Cirromorphida) and the superfamily Argonautoidea are paraphyletic and are broken up; these names are shown in quotation marks and italics on the cladogram.
| Octopoda |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
RNA editing
Octopuses and other coleoid cephalopods are capable of greater RNA editing (which involves changes to the nucleic acid sequence of the primary transcript of RNA molecules) than any other organisms. Editing is concentrated in the nervous system and affects proteins involved in neural excitability and neuronal morphology. More than 60% of RNA transcripts for coleoid brains are recoded by editing, compared to less than 1% for a human or fruit fly. Coleoids rely mostly on ADAR enzymes for RNA editing, which requires large double-stranded RNA structures to flank the editing sites. Both the structures and editing sites are conserved in the coleoid genome and the mutation rates for the sites are severely hampered. Hence, greater transcriptome plasticity has come at the cost of slower genome evolution. High levels of RNA editing do not appear to be present in more basal cephalopods or other molluscs.[128][129]
Relationship to humans

Cultural references
Ancient seafaring people were aware of the octopus, as evidenced by certain artworks and designs. For example, a stone carving found in the archaeological recovery from Bronze Age Minoan Crete at Knossos (1900–1100 BC) has a depiction of a fisherman carrying an octopus.[130] The terrifyingly powerful Gorgon of Greek mythology has been thought to have been inspired by the octopus or squid, the octopus itself representing the severed head of Medusa, the beak as the protruding tongue and fangs, and its tentacles as the snakes.[131] The Kraken are legendary sea monsters of giant proportions said to dwell off the coasts of Norway and Greenland, usually portrayed in art as a giant octopus attacking ships. Linnaeus included it in the first edition of his 1735 Systema Naturae.[132][133] One translation of the Hawaiian creation myth the Kumulipo suggests that the octopus is the lone survivor of a previous age.[134][135][136] The Akkorokamui is a gigantic octopus-like monster from Ainu folklore.[137]

A battle with an octopus plays a significant role in Victor Hugo's book Travailleurs de la mer (Toilers of the Sea), relating to his time in exile on Guernsey.[138] Ian Fleming's 1966 short story collection Octopussy and The Living Daylights, and the 1983 James Bond film were partly inspired by Hugo's book.[139]
Japanese erotic art, shunga, includes ukiyo-e woodblock prints such as Katsushika Hokusai's 1814 print Tako to ama (The Dream of the Fisherman's Wife), in which an ama diver is sexually intertwined with a large and a small octopus.[140][141] The print is a forerunner of tentacle erotica.[142] The biologist P. Z. Myers noted in his science blog, Pharyngula, that octopuses appear in "extraordinary" graphic illustrations involving women, tentacles, and bare breasts.[143][144]
Since it has numerous arms emanating from a common centre, the octopus is often used as a symbol for a powerful and manipulative organisation.[145]
Danger

Octopuses generally avoid humans, but incidents have been verified. For example, a 2.4-metre (8 ft) Pacific octopus, said to be nearly perfectly camouflaged, "lunged" at a diver and "wrangled" over his camera before it let go. Another diver recorded the encounter on video.[146]
All species are venomous, but only blue-ringed octopuses have venom that is lethal to humans.[147] Bites are reported each year across the animals' range from Australia to the eastern Indo-Pacific Ocean. They bite only when provoked or accidentally stepped upon; bites are small and usually painless. The venom appears to be able to penetrate the skin without a puncture, given prolonged contact. It contains tetrodotoxin, which causes paralysis by blocking the transmission of nerve impulses to the muscles. This causes death by respiratory failure leading to cerebral anoxia. No antidote is known, but if breathing can be kept going artificially, patients recover within 24 hours.[148][149] Bites have been recorded from captive octopuses of other species; they leave swellings which disappear in a day or two.[150]
Fisheries and cuisine
Octopus fisheries exist around the world with total catches varying between 245,320 and 322,999 metric tons from 1986 to 1995.[151] The world catch peaked in 2007 at 380,000 tons, and fell by a tenth by 2012.[152] Methods to capture octopuses include pots, traps, trawls, snares, drift fishing, spearing, hooking and hand collection.[151] Octopus is eaten in many cultures and is a common food on the Mediterranean and Asian coasts.[153][154] The arms and sometimes other body parts are prepared in various ways, often varying by species or geography. Live octopuses are eaten in several countries around the world, including the US.[155][156] Animal welfare groups have objected to this practice on the basis that octopuses can experience pain.[157] Octopuses have a food conversion efficiency greater than that of chickens, making octopus aquaculture a possibility.[158]
In science and technology
In classical Greece, Aristotle (384–322 BC) commented on the colour-changing abilities of the octopus, both for camouflage and for signalling, in his Historia animalium: "The octopus ... seeks its prey by so changing its colour as to render it like the colour of the stones adjacent to it; it does so also when alarmed."[159] Aristotle noted that the octopus had a hectocotyl arm and suggested it might be used in sexual reproduction. This claim was widely disbelieved until the 19th century. It was described in 1829 by the French zoologist Georges Cuvier, who supposed it to be a parasitic worm, naming it as a new species, Hectocotylus octopodis.[160][161] Other zoologists thought it a spermatophore; the German zoologist Heinrich Müller believed it was "designed" to detach during copulation. In 1856 the Danish zoologist Japetus Steenstrup demonstrated that it is used to transfer sperm, and only rarely detaches.[162]
Octopuses offer many possibilities in biological research, including their ability to regenerate limbs, change the colour of their skin, behave intelligently with a distributed nervous system, and make use of 168 kinds of protocadherins (humans have 58), the proteins that guide the connections neurons make with each other. The California two-spot octopus has had its genome sequenced, allowing exploration of its molecular adaptations.[163] Having independently evolved mammal-like intelligence, octopuses have been compared to hypothetical intelligent extraterrestrials.[164] Their problem-solving skills, along with their mobility and lack of rigid structure enable them to escape from supposedly secure tanks in laboratories and public aquariums.[165]
Due to their intelligence, octopuses are listed in some countries as experimental animals on which surgery may not be performed without anesthesia, a protection usually extended only to vertebrates. In the UK from 1993 to 2012, the common octopus (Octopus vulgaris) was the only invertebrate protected under the Animals (Scientific Procedures) Act 1986.[166] In 2012, this legislation was extended to include all cephalopods[167] in accordance with a general EU directive.[168]
Some robotics research is exploring biomimicry of octopus features. Octopus arms can move and sense largely autonomously without intervention from the animal's central nervous system. In 2015 a team in Italy built soft-bodied robots able to crawl and swim, requiring only minimal computation.[169] In 2017 a German company made an arm with a soft pneumatically controlled silicone gripper fitted with two rows of suckers. It is able to grasp objects such as a metal tube, a magazine, or a ball, and to fill a glass by pouring water from a bottle.[170]
References
- "ITIS Report: Octopoda Leach, 1818". Itis.gov. 10 April 2013. Retrieved 4 February 2014.
- "Coleoidea – Recent cephalopods". Mikko's Phylogeny Archive.
- Harper, Douglas. "octopus". Online Etymology Dictionary.
- "Octopus". Dictionary.reference.com. Retrieved 4 February 2014.
- ὀκτάπους, ὀκτώπους. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- Michel, Jean-Baptiste; Shen, Yuan; Aiden, Aviva; Veres, Adrian; Gray, Matthew; Pickett, Joseph; Hoiberg, Dale; Clancy, Dan; Norvig, Peter; Orwant, Jon; Pinker, Steven; Nowak, Martin; The Google Books Team (2011). "Quantitative Analysis of Culture Using Millions of Digitized Books". Science. 331 (6014): 176–182. doi:10.1126/science.1199644. PMC 3279742. PMID 21163965. Relevant data at Google Ngram Viewer.
- "Octopus". Oxforddictionaries.com. 2014. Retrieved 4 February 2014.
- Peters, Pam (2004). The Cambridge Guide to English Usage. Cambridge: Cambridge University Press. ISBN 0-521-62181-X, p. 388.
- Fowler, Henry Watson (1994). A Dictionary of Modern English Usage. p. 316. ISBN 9781853263187.
In Latin plurals there are some traps for non-Latinists; the termination of the singular is no sure guide to that of the plural. Most Latin words in -us have plural in -i, but not all, & so zeal not according to knowledge issues in such oddities as...octopi...; as caution the following list may be useful:...octopus, -podes
- Butterfield, Jeremy (2015). Fowler's Dictionary of Modern English Usage. Oxford University Press. ISBN 9780191744532.
The only correct plural in English is octopuses. The Greek original is ὀκτώπους, -ποδ- (which would lead to a pedantic English pl. form octopodes). The pl. form octopi, which is occasionally heard (mostly in jocular use), though based on modL octopus, is misconceived
- Chambers 21st Century Dictionary Archived 24 November 2007 at the Wayback Machine (retrieved 19 October 2007 )
- Stamper, Kory. Ask the editor: octopus. Merriam-Webster. Retrieved 26 June 2013.
- "octopus". Oxford English Dictionary (3rd ed.). Oxford University Press. September 2005. (Subscription or UK public library membership required.)
- Stevenson, Angus; Lindberg, Christine A., eds. (2010). New Oxford American Dictionary (3rd ed.). Oxford University Press. ISBN 978-0-19-539288-3.
- "Smithsonian National Zoological Park: Giant Pacific Octopus". Nationalzoo.si.edu. Archived from the original on 23 February 2014. Retrieved 4 February 2014.
- Cosgrove, J.A. 1987. Aspects of the Natural History of Octopus dofleini, the Giant Pacific Octopus. MSc Thesis. Department of Biology, University of Victoria (Canada), 101 pp.
- Norman, M. 2000. Cephalopods: A World Guide. ConchBooks, Hackenheim. p. 214.
- High, William L. (1976). "The giant Pacific octopus" (PDF). Marine Fisheries Review. 38 (9): 17–22.
- O'Shea, S. (2004). "The giant octopus Haliphron atlanticus (Mollusca : Octopoda) in New Zealand waters". New Zealand Journal of Zoology. 31 (1): 7–13. doi:10.1080/03014223.2004.9518353.
- O'Shea, S. (2002). "Haliphron atlanticus – a giant gelatinous octopus" (PDF). Biodiversity Update. 5: 1.
- Bradford, Alina (21 July 2016). "Octopus Facts". Live Science. Retrieved 26 April 2017.
- Ruppert, Edward E.; Fox, Richard S.; Barnes, Robert D. (2008). Invertebrate Zoology. Cengage Learning. pp. 363–364. ISBN 978-81-315-0104-7.
- Wells (1978), pp. 11–12.
- Ruth A., Byrne; Kuba, Michael J.; Meisel, Daniela V.; Griebel, Ulrike; Mather, Jennifer A. (August 2006). "Does Octopus vulgaris have preferred arms?". Journal of Comparative Psychology. 120 (3): 198–204. doi:10.1037/0735-7036.120.3.198. PMID 16893257.
- Lloyd, John; Mitchinson, John (2010). QI: The Second Book of General Ignorance. London: Faber and Faber. p. 3. ISBN 978-0571273751.
As result, marine biologists tend to refer to them as animals with two legs and six arms.
- Mather, Anderson & Wood (2010), pp. 13–15.
- Courage (2013), pp. 40–41.
- Semmens (2004). "Understanding octopus growth: patterns, variability and physiology". Marine and Freshwater Research. 55 (4): 367. doi:10.1071/MF03155.
- Carefoot, Thomas. "Octopuses and Relatives: Locomotion, Crawling". A Snail's Odyssey. Archived from the original on 22 May 2013. Retrieved 19 April 2017.
- Zelman, I.; Titon, M.; Yekutieli, Y.; Hanassy, S.; Hochner, B.; Flash, T. (2013). "Kinematic decomposition and classification of octopus arm movements". Frontiers in Computational Neuroscience. 7: 60. doi:10.3389/fncom.2013.00060. PMC 3662989. PMID 23745113.
- Tramacere, F.; Beccai, L.; Kuba, M.; Gozzi, A.; Bifone, A.; Mazzolai, B. (2013). "The morphology and adhesion mechanism of Octopus vulgaris suckers". PLOS ONE. 8 (6): e65074. doi:10.1371/journal.pone.0065074. PMC 3672162. PMID 23750233.
- Kier, W. M.; Smith, A. M. (2002). "The structure and adhesive mechanism of octopus suckers". Integrative and Comparative Biology. 42 (6): 1146–1153. CiteSeerX 10.1.1.512.2605. doi:10.1093/icb/42.6.1146. PMID 21680399.
- "Finned Deep-sea Octopuses, Grimpoteuthis spp". MarineBio. Retrieved 25 May 2017.
- Marshall Cavendish Corporation (2004). Encyclopedia of the Aquatic World. Marshall Cavendish. p. 764. ISBN 978-0-7614-7424-1.
- Wells (1978), pp. 31–35.
- Courage (2013), pp. 42–43.
- Schmidt-Nielsen, Knut (1997). Animal Physiology: Adaptation and Environment. Cambridge University Press. p. 117. ISBN 978-0-521-57098-5.
- Carefoot, Thomas. "Octopuses and Relatives: Locomotion, jet propulsion". A Snail's Odyssey. Archived from the original on 28 April 2017. Retrieved 26 April 2017.
- Wells (1978), pp. 24–26.
- Wells, M. J.; Wells, J. (1995). "The control of ventilatory and cardiac responses to changes in ambient oxygen tension and oxygen demand in Octopus". The Journal of Experimental Biology. 198 (Pt 8): 1717–1727. PMID 9319626.
- Wells, J. (1996). "Cutaneous respiration in Octopus vulgaris". The Journal of Experimental Biology. 199 (Pt 11): 2477–2483. PMID 9320405.
- Wells (1978), pp. 73–79.
- Boyle, P. R. (2013). "Neural Control of Cephalopod Behavior". In Dennis Willows, A.O. (ed.). The Mollusca, Volume 8: Neurobiology and Behavior, Part 2. Academic Press. ISBN 978-0-12-751409-3.
- Wells (1978), pp. 54–56.
- Pilleri, Georg (1984). Investigations on Cetacea. 16–17. Hirnanatomisches Institut der Universität. p. 161. Retrieved 30 July 2018.
- NOVA: Kings of camouflage. Film Finance Corporation Australia Limited & Kaufmann Productions; WGBH. 2007.
- Hochner, B. (2012). "An Embodied View of Octopus Neurobiology". Current Biology. 22 (20): R887–R892. doi:10.1016/j.cub.2012.09.001. PMID 23098601.
- Yekutieli, Y.; Sagiv-Zohar, R.; Aharonov, R.; Engel, Y.; Hochner, B.; Flash, T. (2005). "Dynamic model of the octopus arm. I. Biomechanics of the octopus reaching movement". J. Neurophysiol. 94 (2): 1443–1458. doi:10.1152/jn.00684.2004. PMID 15829594.
- Zullo, L.; Sumbre, G.; Agnisola, C.; Flash, T.; Hochner, B. (2009). "Nonsomatotopic organization of the higher motor centers in Octopus". Current Biology. 19 (19): 1632–1636. doi:10.1016/j.cub.2009.07.067. PMID 19765993.
- Kawamura, G.; et al. (2001). "Color Discrimination Conditioning in Two Octopus Octopus aegina and O. vulgaris" (PDF). Nippon Suisan Gakkashi. 67 (1): 35–39. doi:10.2331/suisan.67.35. Archived from the original (PDF) on 14 July 2010.
- "Octopus vision, it's in the eye (or skin) of the beholder". thedishonscience.stanford.edu.
- Harvard University. "Study proposes explanation for how cephalopods see colour, despite black and white vision". Phys.org.
- Sanders, Robert (6 July 2016). "Odd pupils let 'colorblind' octopuses see colors". Futurity.
- Walker, Matt (15 June 2009). "The cephalopods can hear you". BBC. Retrieved 19 July 2013.
- Greenfieldboyce, Nell (15 May 2014). "Why This Octopus Isn't Stuck-Up". NPR.org.
- Wells (1978), pp. 228–244.
- Mather, Anderson & Wood (2010), p. 107.
- Derby, C. D. (2014). "Cephalopod Ink: Production, Chemistry, Functions and Applications". Marine Drugs. 12 (5): 2700–2730. doi:10.3390/md12052700. PMC 4052311. PMID 24824020.
- Mather, Anderson & Wood (2010), p. 147.
- Wells, Martin J.; Wells, J. (1972). "Optic glands and the state of the testis in Octopus". Marine Behaviour and Physiology. 1 (1–4): 71–83. doi:10.1080/10236247209386890.
- Young, R. E.; Vecchione, M.; Mangold, K. M. (1999). "Cephalopoda Glossary". Tree of Life web project.
- Carefoot, Thomas. "Octopuses and Relatives: Reproduction". A Snail's Odyssey. Archived from the original on 22 April 2017. Retrieved 11 April 2017.
- "Giant Pacific Octopus (Enteroctopus dofleini) Care Manual" (PDF). AZA (Association of Zoos and Aquariums) Aquatic Invertebrate Taxonomic Advisory Group in association with AZA Animal Welfare Committee. 9 September 2014. Retrieved 31 May 2016.
- Scheel, David. "Giant Octopus: Fact Sheet". Alaska Pacific University. Archived from the original on 15 November 2012. Retrieved 9 April 2017.
- Anderson, Roland C.; Mather, Jennifer A.; Wood, James B. (2013). Octopus: The Ocean's Intelligent Invertebrate. Timber Press. p. 147. ISBN 978-1-60469-500-7.
- Forsythe, J. W.; Hanlon, R. T. (1980). "A closed marine culture system for rearing Octopus joubini and other large-egged benthic octopods". Laboratory Animals. 14 (2): 137–142. doi:10.1258/002367780780942737. PMID 7431823.
- "Octopus Fact Sheet" (PDF). World Animal Foundation. Retrieved 12 April 2017.
- Simon, Matt (16 January 2015). "Absurd Creature of the Week: The Beautiful Octopus Whose Sex Is All About Dismemberment". Wired: Science. Retrieved 20 May 2017.
- Rowan Hooper (21 December 2019). "Octopuses were thought to be solitary until a social species turned up". New Scientist.
- Anderson, Roland C.; Wood, James B.; Byrne, Ruth A. (2002). "Octopus Senescence: The Beginning of the End". Journal of Applied Animal Welfare Science. 5 (4): 275–283. CiteSeerX 10.1.1.567.3108. doi:10.1207/S15327604JAWS0504_02. PMID 16221078.
- Wodinsky, Jerome (1977). "Hormonal Inhibition of Feeding and Death in Octopus: Control by Optic Gland Secretion". Science. 198 (4320): 948–951. doi:10.1126/science.198.4320.948. PMID 17787564.
- Jamieson, A.J.; M. Vecchione (2020). "First in situ observation of Cephalopoda at hadal depths (Octopoda: Opisthoteuthidae: Grimpoteuthis sp.)". Marine Biology. 167 (82). doi:10.1007/s00227-020-03701-1.
- Norman, Mark (16 January 2013). "Ask an expert: Are there any freshwater cephalopods?". ABC Science. Retrieved 26 April 2017.
- Edmonds, Patricia (April 2016). "What's Odd About That Octopus? It's Mating Beak to Beak". National Geographic.
- Scheel, D.; et al. (2017). "A second site occupied by Octopus tetricus at high densities, with notes on their ecology and behavior". Marine and Freshwater Behaviour and Physiology. 50 (4): 285–291. doi:10.1080/10236244.2017.1369851.
- Goldman, Jason G. (24 May 2012). "How do octopuses navigate?". Scientific American. Retrieved 8 June 2017.
- Courage (2013), pp. 45–46.
- Carefoot, Thomas. "Octopuses and Relatives: Feeding, diets and growth". A Snail's Odyssey. Archived from the original on 8 May 2017. Retrieved 13 April 2017.
- Wassilieff, Maggy; O'Shea, Steve (2 March 2009). "Octopus and squid – Feeding and predation". Te Ara – the Encyclopedia of New Zealand.
- Wells (1978), pp. 74–75.
- Wodinsky, Jerome (1969). "Penetration of the Shell and Feeding on Gastropods by Octopus" (PDF). American Zoologist. 9 (3): 997–1010. doi:10.1093/icb/9.3.997.
- Carefoot, Thomas. "Octopuses and Relatives: Prey handling and drilling". A Snail's Odyssey. Archived from the original on 6 June 2017. Retrieved 21 April 2017.
- Johnsen, S.; Balser, E. J.; Fisher, E. C.; Widder, E. A. (1999). "Bioluminescence in the deep-sea cirrate octopod Stauroteuthis syrtensis Verrill (Mollusca: Cephalopoda)" (PDF). The Biological Bulletin. 197 (1): 26–39. doi:10.2307/1542994. JSTOR 1542994. PMID 28296499. Archived from the original (PDF) on 5 March 2011.
- Huffard, Christine L. (2006). "Locomotion by Abdopus aculeatus (Cephalopoda: Octopodidae): walking the line between primary and secondary defenses". Journal of Experimental Biology. 209 (Pt 19): 3697–3707. doi:10.1242/jeb.02435. PMID 16985187.
- Kassim, I.; Phee, L.; Ng, W. S.; Gong, F.; Dario, P.; Mosse, C. A. (2006). "Locomotion techniques for robotic colonoscopy". IEEE Engineering in Medicine and Biology Magazine. 25 (3): 40–56. doi:10.1109/MEMB.2006.1636351. PMID 16764431.
- Huffard, C. L.; Boneka, F.; Full, R. J. (2005). "Underwater Bipedal Locomotion by Octopuses in Disguise". Science. 307 (5717): 1927. doi:10.1126/science.1109616. PMID 15790846.
- Octopuses have only six arms, the other two are actually legs! Hindustan Times, 13 August 2008.
- Wood, J. B; Anderson, R. C (2004). "Interspecific Evaluation of Octopus Escape Behavior" (PDF). Journal of Applied Animal Welfare Science. 7 (2): 95–106. CiteSeerX 10.1.1.552.5888. doi:10.1207/s15327604jaws0702_2. PMID 15234886. Retrieved 11 September 2015.
- Harmon, Katherine (24 November 2011). "Land-Walking Octopus Explained". Octopus Chronicles. Scientific American. Retrieved 24 November 2011.
- Finn, J. K.; Tregenza, T.; Norman, M. D. (2009). "Defensive tool use in a coconut-carrying octopus". Current Biology. 19 (23): R1069–70. doi:10.1016/j.cub.2009.10.052. PMID 20064403.
- Hamilton, Garry. "What is this octopus thinking?". Archived from the original on 7 April 2012.
- Stewart, Doug (1997). "Armed but not dangerous: Is the octopus really the invertebrate intellect of the sea". National Wildlife. 35 (2).
- "Giant Octopus – Mighty but Secretive Denizen of the Deep". Smithsonian National Zoological Park. 2 January 2008. Archived from the original on 25 August 2012. Retrieved 4 February 2014.
- Zimmer, Carl (23 June 2008). "How Smart is the Octopus?". Slate.com.
- "Octopus intelligence: Jar opening". BBC News. 25 February 2003. Retrieved 4 February 2014.
- Mather, J. A.; Anderson, R. C. (1998). Wood, J. B. (ed.). "What behavior can we expect of octopuses?". The Cephalopod Page.
- Lee, Henry (1875). "V: The octopus out of water". Aquarium Notes – The Octopus; or, the "devil-fish" of fiction and of fact. London: Chapman and Hall. pp. 38–39. OCLC 1544491. Retrieved 11 September 2015.
The marauding rascal had occasionally issued from the water in his tank, and clambered up the rocks, and over the wall into the next one; there he had helped himself to a young lump-fish, and, having devoured it, returned demurely to his own quarters by the same route, with well-filled stomach and contented mind.
- Roy, Eleanor Ainge (14 April 2016). "The great escape: Inky the octopus legs it to freedom from aquarium". The Guardian (Australia).
- Morelle, Rebecca (14 December 2009). "Octopus snatches coconut and runs". BBC News. Retrieved 20 May 2010.
- "Coconut shelter: evidence of tool use by octopuses". EduTube Educational Videos. Archived from the original on 24 October 2013. Retrieved 4 February 2014.
- Meyers, Nadia. "Tales from the Cryptic: The Common Atlantic Octopus". Southeastern Regional Taxonomic Centre. Retrieved 27 July 2006.
- Mather, Anderson & Wood (2010), pp. 90–97.
- https://www.youtube.com/watch?v=-m6CMf1bPkA
- Carefoot, Thomas. "Octopuses and Relatives: Predators and Defenses". A Snail's Odyssey. Archived from the original on 21 April 2017. Retrieved 13 April 2017.
- Mäthger, L. M.; Bell, G. R.; Kuzirian, A. M.; Allen, J. J.; Hanlon, R. T. (2012). "How does the blue-ringed octopus (Hapalochlaena lunulata) flash its blue rings?". Journal of Experimental Biology. 215 (21): 3752–3757. doi:10.1242/jeb.076869. PMID 23053367.
- Wigton, Rachel; Wood, James B. "Grass octopus (Octopus macropus)". Marine Invertebrates of Bermuda. Bermuda Institute of Ocean Sciences. Archived from the original on 19 January 2016. Retrieved 10 August 2018.
- Hanlon, R. T.; Messenger, J. B. (1998). Cephalopod Behaviour. Cambridge University Press. pp. 80–81. ISBN 978-0-521-64583-6.
- Caldwell, R. L. (2005). "An Observation of Inking Behavior Protecting Adult Octopus bocki from Predation by Green Turtle (Chelonia mydas) Hatchlings" (PDF). Pacific Science. 59 (1): 69–72. doi:10.1353/psc.2005.0004. hdl:10125/24161.
- Harmon, Katherine (27 August 2013). "Even Severed Octopus Arms Have Smart Moves". Octopus Chronicles. Scientific American.
- Mather, Anderson & Wood (2010), p. 85.
- Norman, M. D.; Finn, J.; Tregenza, T. (2001). "Dynamic mimicry in an Indo-Malayan octopus" (PDF). Proceedings of the Royal Society. 268 (1478): 1755–8. doi:10.1098/rspb.2001.1708. PMC 1088805. PMID 11522192. Archived from the original (PDF) on 10 February 2012. Retrieved 1 October 2008.
- Norman, M. D. (2005). "The 'Mimic Octopus' (Thaumoctopus mimicus n. gen. et sp.), a new octopus from the tropical Indo-West Pacific (Cephalopoda: Octopodidae)". Molluscan Research. 25 (2): 57–70.
- Pascal, Santiago; Gestal, Camino; Estevez, J.; Arias, Christian Andrés (1996). "Parasites in commercially-exploited cephalopods (Mollusca, Cephalopoda) in Spain: An updated perspective". Aquaculture. 142 (1–2): 1–10. doi:10.1016/0044-8486(96)01254-9.
- Furuya, Hidetaka; Tsuneki, Kazuhiko (2003). "Biology of Dicyemid Mesozoans". Zoological Science. 20 (5): 519–532. doi:10.2108/zsj.20.519. PMID 12777824.
- Castellanos-Martínez, Sheila; Gestal, Camino (2013). "Pathogens and immune response of cephalopods". Journal of Experimental Marine Biology and Ecology. 447: 14–22. doi:10.1016/j.jembe.2013.02.007.
- Farto, R.; Armada, S. P.; Montes, M.; Guisande, J. A.; Pérez, M. J.; Nieto, T. P. (2003). "Vibrio lentus associated with diseased wild octopus (Octopus vulgaris)". Journal of Invertebrate Pathology. 83 (2): 149–156. doi:10.1016/S0022-2011(03)00067-3. PMID 12788284.
- Gofas, S. (2009). "Octopoda". WoRMS. World Register of Marine Species. Retrieved 5 May 2017.
- Mather, Anderson & Wood (2010), p. 145.
- Sanchez, Gustavo; Setiamarga, Davin H. E.; Tuanapaya, Surangkana; Tongtherm, Kittichai; Winkelmann, Inger E.; Schmidbaur, Hannah; Umino, Tetsuya; Albertin, Caroline; Allcock, Louise; Perales-Raya, Catalina; Gleadall, Ian; Strugnell, Jan M.; Simakov, Oleg; Nabhitabhata, Jaruwat (2018). "Genus-level phylogeny of cephalopods using molecular markers: current status and problematic areas". PeerJ. 6: e4331. doi:10.7717/peerj.4331. PMC 5813590. PMID 29456885.
- Fuchs, D.; Ifrim, C.; Stinnesbeck, W. (2008). "A new Palaeoctopus (Cephalopoda: Coleoidea) from the Late Cretaceous of Vallecillo, north-eastern Mexico, and implications for the evolution of Octopoda". Palaeontology. 51 (5): 1129–1139. doi:10.1111/j.1475-4983.2008.00797.x.
- Philippe Bouchet (2015). "Octopodoidea d'Orbigny, 1840". World Register of Marine Species. Flanders Marine Institute. Retrieved 3 February 2018.
- Courage (2013), p. 4.
- "A Broad Brush History of the Cephalopoda". The Cephalopod Group. Retrieved 27 March 2017.
- Young, R. E.; Vecchione, M.; Mangold, K. M. (1999). "Cephalopoda Glossary". Tree of Life web project. Retrieved 30 May 2017.
- "Octopuses & Squids". Vancouver Aquarium. Retrieved 29 May 2017.
- Norman, M. (2000). Cephalopods: A World Guide. ConchBooks. p. 15. ISBN 978-3-925919-32-9.
- Seibel, B. "Vampyroteuthis infernalis, Deep-sea Vampire squid". The Cephalopod Page. Retrieved 31 May 2017.
- Courage (2013), pp. 46–49.
- Liscovitch-Brauer, N.; Alon, S.; Porath, H. T.; Elstein, B.; Unger, R.; Ziv, T.; Admon, A.; Levanon, E. Y.; Rosenthal, J. J. C.; Eisenberg, E. (2017). "Trade-off between transcriptome plasticity and genome evolution in cephalopods". Cell. 169 (2): 191–202. doi:10.1016/j.cell.2017.03.025. PMC 5499236. PMID 28388405.
- Hogan, C. Michael (22 December 2007). "Knossos fieldnotes". The Modern Antiquarian.
- Wilk, Stephen R. (2000). Medusa: Solving the Mystery of the Gorgon. Oxford University Press. ISBN 978-0-19-988773-6.
- "Caroli Linnaei Systema naturae sistens regna tria naturae". google.com.
- Smedley, Edward; Rose, Hugh James; Rose, Henry John (1845). Encyclopaedia Metropolitana, Or, Universal Dictionary of Knowledge: Comprising the Twofold Advantage of a Philosophical and an Alphabetical Arrangement, with Appropriate Engravings. B. Fellowes. pp. 255–.
- Dixon, Roland Burrage (1916). Oceanic. The Mythology of All Races. 9. Marshall Jones Company. pp. 2–.
- Bastian, Adolf (1881). Die heilige Sage der Polynesier: Kosmogonie und Theogonie. Oxford University. Leipzig: F. A. Brockhaus. pp. 107–108.
- Beckwith, Martha Warren (1981). The Kumulipo: A Hawaiian Creation Chant. University of Hawaii Press. pp. 52–53. ISBN 978-0824807719.
- Batchelor, John (1901). The Ainu and Their Folklore. London: The Religious Tract Society.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica (11th ed.). Cambridge University Press.
- Cohen-Vrignaud, Gerard (2012). "On Octopussies, or the Anatomy of Female Power". Differences. 23 (2): 32–61. doi:10.1215/10407391-1533520.
- Fritze, Sointu; Suojoki, Saara (2000). Forbidden Images: Erotic Art from Japan's Edo Period (in Finnish). Helsingin kaupungin taidemuseo. pp. 23–28. ISBN 978-951-8965-54-4.
- Uhlenbeck, Chris; Margarita Winkel; Ellis Tinios; Amy Reigle Newland (2005). Japanese Erotic Fantasies: Sexual Imagery of the Edo Period. Hotei. p. 161. ISBN 978-90-74822-66-4.
- Briel, Holger (2010). Berninger, Mark; Ecke, Jochen; Haberkorn, Gideon (eds.). The Roving Eye Meets Traveling Pictures: The Field of Vision and the Global Rise of Adult Manga. Comics As a Nexus of Cultures: Essays on the Interplay of Media, Disciplines. McFarland. p. 203. ISBN 978-0-7864-3987-4.
- Myers, P. Z. (17 May 2017). "Extraordinary Octopus Illustrations". Pharyngula. Retrieved 18 March 2017.
- Myers, P. Z. (29 October 2006). "Definitely not safe for work". Pharyngula. Retrieved 18 March 2017.
- Smith, S. (26 February 2010). "Why Mark Zuckerberg Octopus Cartoon Evokes 'Nazi Propaganda,' German Paper Apologizes". iMediaEthics. Retrieved 31 May 2017.
- Ross, Philip (18 February 2014). "8-Foot Octopus Wrestles Diver Off California Coast, Rare Encounter Caught on Camera". International Business Times.
- "Tentacles of venom: new study reveals all octopuses are venomous". University of Melbourne. 15 April 2009.
- "Blue-ringed Octopuses, Hapalochlaena maculosa". The MarineBio Conservation Society. Archived from the original on 16 February 2017. Retrieved 12 April 2017.
- Caldwell, Roy. "What makes blue-rings so deadly? Blue-ringed octopus have tetrodotoxin". The Cephalopod Page. Retrieved 12 April 2017.
- Wells (1978), pp. 68.
- Gillespie, G. E.; Parker, G.; Morrison, J. (1998). "A Review of Octopus Fisheries Biology and British Columbia Octopus Fisheries" (PDF). Canadian Stock Assessment Secretariat.
- Rocliffe, S.; Harris, A. (2016). "The status of octopus fisheries in the Western Indian Ocean". Retrieved 18 June 2017.
- Cushman, Abi (24 August 2014). "Common octopus". Animal fact guide.
- "Giant Pacific octopus". Monterey Bay Aquarium. 2017.
- Eriksen, L. (10 November 2010). "Live and let dine". The Guardian. Retrieved 15 April 2015.
- Killingsworth, Silvia (3 October 2014). "Why not eat octopus?". The New Yorker. Retrieved 15 April 2016.
- Ferrier, M. (30 May 2010). "Macho foodies in New York develop a taste for notoriety". The Guardian. Retrieved 15 April 2015.
- Wells, Martin (1983). "Cephalopods do it differently". New Scientist. 100 (1382): 333–334. ISSN 0262-4079.
- Aristotle (c. 350 BC). Historia animalium. IX, 622a: 2–10. Cited in Borrelli, Luciana; Gherardi, Francesca; Fiorito, Graziano (2006). A catalogue of body patterning in Cephalopoda. Firenze University Press. ISBN 978-88-8453-377-7. Abstract
- Leroi, Armand Marie (2014). The Lagoon: How Aristotle Invented Science. Bloomsbury. pp. 71–72. ISBN 978-1-4088-3622-4.
- "The Cephalopoda". University of California Museum of Paleontology. Retrieved 27 March 2017.
- Mann, T. (2012). Spermatophores: Development, Structure, Biochemical Attributes and Role in the Transfer of Spermatozoa. Springer. p. 28. ISBN 978-3-642-82308-4.
- Singer, Emily (26 July 2016). "Biologists Search for New Model Organisms". Quanta Magazine.
- Baer, Drake (20 December 2016). "Octopuses Are 'the Closest We Will Come to Meeting an Intelligent Alien'". Science of Us. Retrieved 26 April 2017.
- Brulliard, Karin (13 April 2016). "Octopus slips out of aquarium tank, crawls across floor, escapes down pipe to ocean". The Washington Post. Retrieved 20 February 2017.
- "The Animals (Scientific Procedures) Act (Amendment) Order 1993". The National Archives. Retrieved 18 February 2015.
- "The Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012". The National Archives. Retrieved 18 February 2015.
- "Directive 2010/63/EU of the European Parliament and of the Council". Article 1, 3(b) (see page 276/39): Official Journal of the European Union. Retrieved 18 February 2015.CS1 maint: location (link)
- "Octopus-Inspired Robots Can Grasp, Crawl, and Swim". IEEE Spectrum. 5 April 2015.
- Burgess, Matt (27 March 2017). "This robotic octopus tentacle isn't creepy at all". Wired.
Bibliography
- Wells, M. J. (1978). Octopus, Physiology and Behaviour of an Advanced Invertebrate. Springer Science & Business Media. ISBN 978-94-017-2470-8.
- Courage, K. H. (2013). Octopus!, The Most Mysterious Creature in the Sea. Penguin Group. ISBN 978-0-698-13767-7.
- Mather, J. A.; Anderson, R. C.; Wood, J. B. (2010). Octopus: The Ocean's Intelligent Invertebrate. Timber Press. ISBN 978-1-60469-067-5.
External links
| The Wikibook Dichotomous Key has a page on the topic of: Octopoda |








.png)

