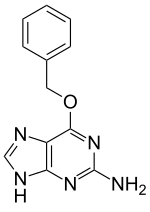
O6-Benzylguanine
O6-Benzylguanine (O6-BG) is a synthetic derivative of guanine. It is an antineoplastic agent. It exerts its effect by acting as a suicide inhibitor of the enzyme O6-alkylguanine-DNA alkyltransferase which leads to interruption of DNA repair. O6-BG was used clinically in combination with the alkylating agent temozolomide for glioblastoma, however the combination was found to be overly toxic without adding significant benefit.[1][2][3]
 | |
| Names | |
|---|---|
| IUPAC name
6-(Benzyloxy)-7H-purin-2-amine | |
| Other names
6-(Benzyloxy)guanine; 6-O-Benzylguanine; O(6)-bGua; O6-BG | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.161.788 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H11N5O | |
| Molar mass | 241.254 g·mol−1 |
| Density | 1.432 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
O6-BG is also used as a biochemical tool in the study of DNA repair mechanisms.
References
- Quinn, JA; Desjardins, A; Weingart, J; Brem, H; Dolan, ME; Delaney, SM; Vredenburgh, J; Rich, J; et al. (2005). "Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma". Journal of Clinical Oncology. 23 (28): 7178–87. doi:10.1200/JCO.2005.06.502. PMID 16192602.
- Quinn, J. A.; Jiang, S. X.; Reardon, D. A.; Desjardins, A.; Vredenburgh, J. J.; Rich, J. N.; Gururangan, S.; Friedman, A. H.; et al. (2009). "Phase II Trial of Temozolomide Plus O6-Benzylguanine in Adults with Recurrent, Temozolomide-Resistant Malignant Glioma". Journal of Clinical Oncology. 27 (8): 1262–7. doi:10.1200/JCO.2008.18.8417. PMC 2667825. PMID 19204199.
- Blumenthal, Deborah T. (2015). "A phase III study of radiation therapy (RT) and O6-benzylguanine, (O6-BG) plus BCNU versus RT and BCNU alone and methylation status in newly-diagnosed glioblastoma (GBM) and gliosarcoma: Southwest Oncology Group (SWOG) Study S0001". International Journal of Clinical Oncology. 20: 650–8. doi:10.1007/s10147-014-0769-0. PMC 4465052. PMID 25407559.
External links
- Friedman, HS (2000). "Can O6-alkylguanine-DNA alkyltransferase depletion enhance alkylator activity in the clinic?" (PDF). Clinical Cancer Research. 6 (8): 2967–8. PMID 10955771.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.