Notheia anomala
Notheia anomala is a macroalgae in the family Notheiceae and the order Fucales. It is native to New Zealand and Australia. It is an obligate epiphyte that is commonly found growing on Hormosira (although there is a single unconfirmed observation of Notheia being attached to Xiphophora chondrophylla).[1][2] Hormosira is also from the order Fucales - and it is rare to have an epiphyte so closely related to its host.
| Notheia anomala | |
|---|---|
 | |
| Notheia specimen found in Kaikoura, New Zealand. Photo taken by I. Metcalfe. | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Notheia |
| Species: | N. anomala |
| Binomial name | |
| Notheia anomala Harvey & J.W.Bailey (1851) | |
It appears Notheia growth is entirely dependent on being attached to Hormosira. For example, attempts to grow Notheia in culture were unsuccessful until Hormosira extracts were added.[3] However, there are few suggestions as to whether Notheia has a parasitic effect on Hormosira.
When Hallam et al. (1980) [3] studied natural populations of Hormosira in Australia they found that tide pool populations had a consistently higher proportion of infected plants (with Notheia) than the low shore reef populations. This suggests that Notheia has a much narrower tolerance limit than its host. They found that sexually mature Hormosira plants carried more infections and the infections were most abundant on the reproductive conceptacles usually close to the osteoles. When looking at the settlement preferences of Notheia, Hallam and colleagues (1980) [3] discovered that it did not show any partiality towards a particular sex of its dioecious host.

Sexual reproduction
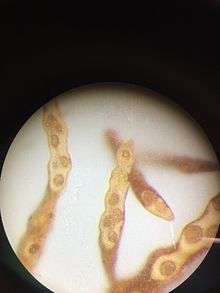
Notheia produces male and female gametangia in the same conceptacle (monoecious).[1][3]
Female gametangia
Female gametangia are present throughout the year, and they develop either on branched or unbranched stalks within the conceptacle, or directly from the walls of conceptacles. They contain 8 zooids each 10-12 µm long by 5-6 µm wide. About 4 hours after release via paraphyses, female gametes begin to settle.
Male gametangia
Male gametangia have been observed only between April and July (Southern Hemisphere, Australia). They are borne in a similar manner to the female gametangia, and contain 64 zooids, each 5 µm long by 2-3 µm wide.
Both gametes are pyriform in shape (pear form) with two laterally inserted flagella (the anterior one longer than the posterior). They each possess one eyespot - and have never been observed to contain two or more. Male and female gametes freely intermingle within a conceptacle, but as long as female gametes are motile, males are not attracted to them. About 4 h after release, female gametes settle on surrounding Hormosira tissue, and once that has occurred, the male gametes are then attracted to the settled female gametes.
Costs and benefits of epiphytism
The costs to Hormosira in this relationship are still unclear. Even though the tissues of Hormosira are pushed up against the thallus of Notheia giving the impression that Notheia is emerging from deep within, there are no plasmodesmata observed between adjacent Notheia and Hormosira cells.[3] Similarly, the benefits that Notheia obtains from attaching to Hormosira are also unclear. The fronds of Hormosira are weakly attached to the substratum and there is frequent dislodgement in storm events or periods of high wave energy – therefore the fronds that drift may offer long-distance dispersal to Notheia which could be a key mechanism for the distributional success of this species[4]
Capon et al. (1998) [5] highlighted for the first time that tetrahydrofurans from Notheia act as potent and selective inhibitors of the larval development of parasitic nematodes, which may be a positive factor that Hormosira receives from this symbiotic relationship.
Scientific interest
Analyses of Notheia biomass have shown a strong positive effect on invertebrate biodiversity.[6] Notheia provides food, protection, and niche space for a large range of small invertebrates, an important group of organisms that again provide food for higher trophic levels. See Thomsen et al. (2016) for details,[6] or Wikipedia search Habitat Cascade. Notheia also has a higher photosynthetic capacity than its host Hormosira, probably because of its greater surface area or smaller allocation to chemical defences.[7] Therefore, when judging ecosystem health, it is important to take these factors into account and consider how epiphytes are contributing to ecosystem productivity.
References
- Gibson, G., & Clayton, M. N. (1987). Sexual reproduction, early development and branching in Notheia anomala (phaeophyta) and its classification in the Fucales. Phycologia, 26(3), 363-373.
- Raven, J. A., Beardall, J., Johnston, A. M., Kuebler, J. E., & McInroy, S. G. (1996). Inorganic carbon acquisition by Xiphophora chondrophylla (Phaeophyta, Fucales). Phycologia, 35(2), 83-89.
- Hallam, N. D., Clayton, M. N., & Parish, D. (1980). Studies on the association between Notheia anomala and Hormosira banksii (Phaeophyta). Australian Journal of Botany, 28(2), 239-248.
- McKenzie, P. F., & Bellgrove, A. (2009). Dislodgment and attachment strength of the intertidal macroalga Hormosira banksii (Fucales, Phaeophyceae). Phycologia, 48(5), 335-343.
- Capon, R. J., Barrow, R. A., Rochfort, S., Jobling, M., Skene, C., Lacey, E., Gill, J. H., Friedel, T., & Wadsworth, D. (1998). Marine nematocides: tetrahydrofurans from a southern Australian brown alga, Notheia anomala. Tetrahedron, 54(10), 2227-2242.
- Thomsen, M. S., Metcalfe, I., South, P., & Schiel, D. R. (2016). A host-specific habitat former controls biodiversity across ecological transitions in a rocky intertidal facilitation cascade. Marine and Freshwater Research, 67(1), 144-152.
- Raven, J., Beardall, J., Johnston, A., Kübler, J., & Geoghegan, I. (1995). Inorganic carbon acquisition by Hormosira banksii (Phaeophyta: Fucales) and its epiphyte Notheia anomala (Phaeophyta: Fucales). Phycologia, 34(4), 267-277.
