Nonaflate
Nonaflate, CF
3CF
2CF
2CF
2SO−
3, is the common name given to nonafluorobutanesulfonates, the salts or esters of perfluorobutanesulfonic acid. Its uses are similar to those of triflate.
It is a good leaving group. It is a substitute for more toxic long-chain PFAS chemicals.[1]
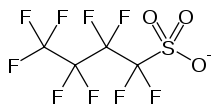
Skeletal formula of the nonaflate anion
References
- Thurlow, Matthew (December 27, 2018). "Fear and loathing of PFAS". American Bar Association. Retrieved 19 December 2019.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.