Nitrile reduction
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.[1][2]
Catalytic hydrogenation
The catalytic hydrogenation of nitriles is often the most economical route available for the production of primary amines.[3] Catalysts for the reaction often include group 10 metals such as Raney nickel,[4][5][6] palladium black, or platinum dioxide.[1] However, other catalysts, such as cobalt boride, also can be selective for primary amine production:
- R-C≡N + 2 H2 → R-CH2NH2
A commercial application of this technology includes the production of hexamethylenediamine from adiponitrile, a precursor to Nylon 66.[7]
Depending on reaction conditions, intermediate imines can also undergo attack by amine products to afford secondary and tertiary amines:
- 2 R-C≡N + 4 H2 → (R-CH2)2NH + NH3
- 3 R-C≡N + 6 H2 → (R-CH2)3N + 2 NH3
Such reactions proceed via enamine intermediates.[8] The most important reaction condition for selective primary amine production is catalyst choice.[1] Other important factors include solvent choice, solution pH, steric effects, temperature, and the pressure of hydrogen.
Stoichiometric reductions
Reducing agents for the non-catalytic conversion to amines include lithium aluminium hydride, lithium borohydride,[9] diborane,[10] or elemental sodium in alcohol solvents.[11]
To aldehydes
Nitriles can also be reduced to aldehydes. The Stephen aldehyde synthesis uses Tin(II) chloride and hydrochloric acid to yield an aldehyde via the hydrolysis of a resulting iminium salt. Aldehydes can also form using a hydrogen donor followed by in-situ hydrolysis of an imine. Useful reagents for this reaction include formic acid with a hydrogenation catalysis[12] or metal hydrides which are used to add one mol of hydrogen to the nitrile. For example, sodium borohydride reduces nitriles in alcoholic solvents with a CoCl2 catalyst or Raney nickel.[13] Reducing agent Diisobutylaluminium hydride, or DIBAL-H, is another commonly used metal hydride. DIBAL-H acts as a proton source, adding a hydride ion to the carbon of the nitrile. The resulting imine is a relatively stable intermediate that can be hydrolyzed to the aldehyde.[14]
Mechanism
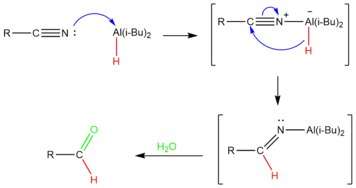
DIBAL-H is added in controlled amounts at low temperatures to achieve partial reduction of the nitrile.[15] The aluminum atom in DIBAL acts as a Lewis acid, accepting an electron pair from the nitrile. The nitrile is then reduced by the transfer of a hydride ion to the carbon of the carbon-nitrile triple bond, producing an imine. After a workup with water, the aluminum complex is hydrolyzed to produce the desired aldehyde.[16] Because the hydrolytic workup generates the aldehyde at the end, the nitrile does not undergo over-reduction.
Electrochemical methods
Benzonitriles can also be reduced electrochemically.[17][18]
See also
References
- Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). Newyork: Wiley-Interscience. pp. 254–277. ISBN 9780471396987.
- March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- Karsten, Eller; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a02_001. ISBN 3527306730.
- Biggs, B. S.; Bishop., W. S. (1947). "Decamethylenediamine". Organic Syntheses. 29: 18. doi:10.15227/orgsyn.027.0018.
- Allen, C. F. H.; Wilson, C. V. (1947). "2,4-Diphenylpyrrole". Organic Syntheses. 27: 33. doi:10.15227/orgsyn.027.0033.
- Robinson, John C.; Snyder, H. R. (1943). "β-Phenylethylamine". Organic Syntheses. 23: 71. doi:10.15227/orgsyn.023.0071.
- Musser, Michael Tuttle (2000). "Adipic Acid". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a01_269. ISBN 3527306730.
- Barrault, J. (1997). "Synthesis of fatty amines. Selectivity control in presence of multifunctional catalysts". Catalysis Today. 37 (2): 137–153. doi:10.1016/S0920-5861(97)00006-0.
- Ookawa, Atsuhiro; Soai, Kenso (1986). "Mixed solvents containing methanol as useful reaction media for unique chemoselective reductions within lithium borohydride". The Journal of Organic Chemistry. 51 (21): 4000–4005. doi:10.1021/jo00371a017.
- Hutchins, R. O.; Maryanoff, B. E. (1973). "2-tert-Butyl-1,3-diaminoproane". Organic Syntheses. 53: 21. doi:10.15227/orgsyn.053.0021.
- Suter, C. M.; Moffett, Eugene W. (1934). "The Reduction of Aliphatic Cyanides and Oximes with Sodium and n-Butyl Alcohol". Journal of the American Chemical Society. 56 (2): 487. doi:10.1021/ja01317a502.
- van Es, T.; Staskun, B. (1971). "4-Formylbenzenesulfonamide". Organic Syntheses. 51: 20. doi:10.15227/orgsyn.051.0020.
- 17-, Smith, Michael, 1946 October (2001). March's advanced organic chemistry : reactions, mechanisms, and structure. March, Jerry, 1929-1997., March, Jerry, 1929-1997. (5th ed.). New York: Wiley. ISBN 9780471585893. OCLC 43936853.CS1 maint: numeric names: authors list (link)
- Carey, F. A.; Sundberg, R. J.; Advanced Organic Chemistry, Part B: Reactions and Synthesis
- 1937-, Carey, Francis A. (2000). Advanced organic chemistry. Sundberg, Richard J., 1938- (4th ed.). New York: Kluwer Academic/Plenum Pub. ISBN 0306462435. OCLC 43555205.CS1 maint: numeric names: authors list (link)
- Solomons, T W. G, Craig B. Fryhle, and S A. Snyder. Organic Chemistry. , 2014. Print.
- V. Krishnan; A. Muthukumaran; H. V. K. Udupa (1979). "The electroreduction of benzyl cyanide on iron and cobalt cathodes". Journal of Applied Electrochemistry. 9 (5): 657–659. doi:10.1007/BF00610957.
- V. Krishnan; A. Muthukumaran; H. V. K. Udupa (1983). Process for Electrochemical Preparation of beta phenylethylamine using cobalt black cathode (PDF). Calcutta: India Patent Office.