Negative hyperconjugation
In organic chemistry, negative hyperconjugation is the donation of electron density from a filled π- or p-orbital to a neighboring σ*-orbital.[1] This phenomenon, a type of resonance, can stabilize the molecule or transition state.[2] It also causes an elongation of the σ-bond by adding electron density to its antibonding orbital.[1]
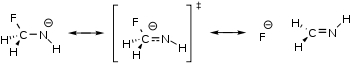
A schematic representation of negative hyperconjugation. In real systems, several of the hydrogens are replaced with other functional groups.
Negative hyperconjugation is most commonly observed when the σ*-orbital is located on certain C–F or C–O bonds,[3][4] and does not occur to an appreciable extent with normal C–H bonds.
In negative hyperconjugation, the electron density flows in the opposite direction (from π- or p-orbital to empty σ*-orbital) than it does in the more common hyperconjugation (from σ-orbital to empty p-orbital).
See also
References
- "Negative Hyper-Conjugation" (PDF). Old.iupac.org. Archived from the original (PDF) on 2012-03-24. Retrieved 2013-10-23.
- "2.2.3 Negative Hyperconjugation for Ebooksclub.org Molecular Orbitals and Organic Chemical Reactions Student Edition". Scribd.com. 2011-04-29. Retrieved 2012-08-20.
- "Negative hyperconjugation of some fluorine containing groups – New Journal of Chemistry (RSC Publishing)". Pubs.rsc.org. Retrieved 2012-08-20.
- Karni, Miriam; Bernasconi, Claude F.; Rappoport, Zvi (2007-08-09). "Role of Negative Hyperconjugation and Anomeric Effects in the Stabilization of the Intermediate in SNV Reactions". The Journal of Organic Chemistry. 73 (8): 2980–2994. doi:10.1021/jo7017476. PMID 18376875.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.