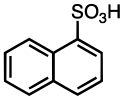
Naphthalene-1-sulfonic acid
Naphthalene-1-sulfonic acid is an organic compound with the formula C10H7SO3H. A colorless, water-soluble solid, it is often available as the dihydrate C10H7SO3H.2H2O. It is one of two monosulfonic acids of naphthalene, the other being the more stable naphthalene-2-sulfonic acid. The compound is mainly used in the production of dyes.[1]
 | |
| Names | |
|---|---|
| Other names
1-naphthalenesulfonic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 2099069 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.464 |
| EC Number |
|
| 366290 | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H8O3S | |
| Molar mass | 208.23 g·mol−1 |
| Appearance | white solid |
| Melting point | 139–140 °C (282–284 °F; 412–413 K) |
| good | |
| Hazards | |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H314, H318, H351, H411 |
| P201, P202, P260, P264, P270, P273, P280, P281, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P308+313, P310, P321, P330, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Naphthalene-1-sulfonic acid undergoes many reactions, some of which are or were of commercial interest. Upon heating with dilute aqueous acid, it reverts to naphthalene. Fusion with sodium hydroxide followed by acidification gives 1-naphthol. Further sulfonation gives 1,5-naphthalene-disulfonic acid. Reduction with triphenylphosphine gives 1-naphthalenethiol.
References
- Gerald Booth (2005). "Naphthalene Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_009.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.