Monomethyl fumarate
Monomethyl fumarate, sold under the brand name Bafiertam is a medication for the treatment of relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Bafiertam |
| License data | |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
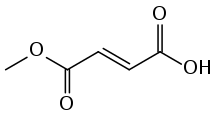
| Formula | C5H6O4 |
| Molar mass | 130.099 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It was approved for medical use in the United States in April 2020.[2]
The most common adverse reactions are flushing, abdominal pain, diarrhea, and nausea.[1]
Names
Monomethyl fumarate is the International Nonproprietary Name (INN).[3]
See also
References
- "Bafiertam (monomethyl fumarate) delayed-release capsules, for oral use" (PDF). U.S. Food and Drug Administration (FDA). Banner Life Sciences. Retrieved 29 April 2020.
- "Bafiertam: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 29 April 2020.
- World Health Organization (2020). "International nonproprietary names for pharmaceutical substances (INN) : recommended INN: list 80" (PDF). WHO Drug Information. 34 (1): 74.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.