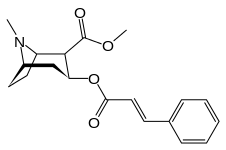
Methylecgonine cinnamate
Methylecgonine cinnamate is a natural tropane alkaloid found within the coca plant.[1] Its more common name, cinnamoylcocaine, reflects its close structural similarity to cocaine. It is said to be pharmacologically inactive.[2] But some studies funded by anti-drug agencies imply that it is active when smoked. Furthermore, the discovery of differing impurity products yielding methylecgonine cinnamate in confiscated cocaine have led enforcing agencies to postulate that illicit manufacturers have changed their oxidation procedures when refining cocaine from a crude form.[3] Methylecgonine cinnamate can dimerize to the truxillic acid derivative truxilline.[2] It is notable that methylecgonine cinnamate is given in patents of active cocaine analogue structures.[4][5]
 | |
| Names | |
|---|---|
| IUPAC name
methyl (1R,2R,3S,5S)-8-methyl-3-[(E)-3-phenylprop-2-enoyl]oxy-8- | |
| Other names
Cinnamoylcocaine Cinnamylcocaine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H23NO4 | |
| Molar mass | 329.396 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Plowman, T.; Rivier, L. (1983). "Cocaine and Cinnamoylcocaine Content of Erythroxylum Species". Annals of Botany. 51 (5): 641–659. doi:10.1093/oxfordjournals.aob.a086511.
- Merck Chemical Index, 1985
- Casale, J. F.; Hays, P. A.; Toske, S. G.; Berrier, A. L. (Jul 2007). "Four new illicit cocaine impurities from the oxidation of crude cocaine base: formation and characterization of the diastereomeric 2,3-dihydroxy-3-phenylpropionylecgonine methyl esters from cis- and trans-cinnamoylcocaine". J Forensic Sci. 52 (4): 860–6. doi:10.1111/j.1556-4029.2007.00476.x. PMID 17553089.
- U.S. Patent 6,479,509 Patent inventor Frank Ivy Carroll, Assignee: Research Triangle Institute
- U.S. patent US6479509 B1 structures given for submission, 5th compound down in image.
External links
- Isolation of cis-Cinnamoylcocaine from Crude Illicit Cocaine via Alumina Column Chromatography D.E.A. Microgram Vol 4 Number 14.
- Rubio, Nelida Cristina; Hastedt, Martin; Gonzalez, Jorge; Pragst, Fritz (2015). "Possibilities for discrimination between chewing of coca leaves and abuse of cocaine by hair analysis including hygrine, cuscohygrine, cinnamoylcocaine and cocaine metabolite/cocaine ratios". International Journal of Legal Medicine. 129 (1): 69–84. doi:10.1007/s00414-014-1061-6. PMID 25138383.