Meniscus (liquid)
A concave meniscus occurs when the particles of the liquid are more strongly attracted to the container (adhesion) than to each other (cohesion), causing the liquid to climb the walls of the container. This occurs between water and glass. Water-based fluids like sap, honey, and milk also have a concave meniscus in glass or other wettable containers.
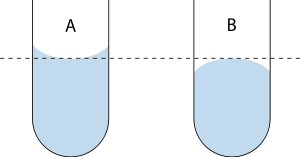
B: The top of a convex meniscus.
Conversely, a convex meniscus occurs when the particles in the liquid have a stronger attraction to each other than to the material of the container.[1] Convex menisci occur, for example, between mercury and glass in barometers[1] and thermometers.

Contact angle and surface tension

The formation of menisci is commonly used in surface science to measure contact angles and surface tension. In a contact angle measurement, the shape of the menisci is measured with a balance or optically with a digital camera. In a surface tension measurement, the measurement probe has a contact angle of zero and the surface tension can be obtained by measuring the mass of the menisci. This is typically done with a Wilhelmy plate.[2]
Capillary action
Menisci are a manifestation of capillary action, by which surface adhesion pulls a liquid up to form a concave meniscus or internal cohesion pulls the liquid down to form a convex meniscus. This phenomenon is important in transpirational pull in plants. When a tube of a narrow bore, often called a capillary tube, is dipped into a liquid and the liquid wets the tube (with zero contact angle), the liquid surface inside the tube forms a concave meniscus, which is a virtually spherical surface having the same radius, r, as the inside of the tube. The tube experiences a downward force of magnitude 2πrσ, where σ is the surface tension of the liquid.[3]
References
- Moore, John W.; Stanitski, Conrad L.; Jurs, Peter C. (2005). Chemistry: The Molecular Science. Belmont, CA: Brooks/Cole. p. 290.
- Biolin Scientific. "Surface and interfacial tension | White Paper".
- "Fluid Mechanics". Encyclopædia Britannica. Retrieved 14 November 2014.