Lysis buffer
A lysis buffer is a buffer solution used for the purpose of breaking open cells for use in molecular biology experiments that analyze the labile macromolecules of the cells (e.g. western blot for protein, or for DNA extraction). Most lysis buffers contain buffering salts (e.g. Tris-HCl) and ionic salts (e.g. NaCl) to regulate the pH and osmolarity of the lysate. Sometimes detergents (such as Triton X-100 or SDS) are added to break up membrane structures. For lysis buffers targeted at protein extraction, protease inhibitors are often included, and in difficult cases may be almost required. Lysis buffers can be used on both animal and plant tissue cells.[1]
Choosing a buffer
The primary purpose of lysis buffer is isolating the molecules of interest and keeping them in a stable environment. For proteins, for some experiments, the target proteins should be completely denatured, while in some other experiments the target protein should remain folded and functional. Different proteins also have different properties and are found in different cellular environments. Thus, it is essential to choose the best buffer based on the purpose and design of the experiments. The important factors to be considered are: pH, ionic strength, usage of detergent, protease inhibitors to prevent proteolytic processes.[2] For example, detergent addition is necessary when lysing Gram-negative bacteria, but not for Gram-positive bacteria.[3] It is common that a protease inhibitor is added to lysis buffer, along with other enzyme inhibitors of choice, such as a phosphatase inhibitor when studying proteins with phosphorylation.
Components
Buffer
Buffer creates an environment for isolated proteins. Each buffer choice has a specific pH range, so the buffer should be chosen based on whether your target protein is stable under a certain pH. Also, for buffers with similar pH ranges, it is important to consider whether the buffer is compatible with your target protein.[4] The table below contains several most commonly used buffers and their pH ranges.[4]
| Buffer | pH Range |
|---|---|
| Sodium dihydrogen phosphate / disodium hydrogen phosphate | 5.8 - 8.0 |
| Tris - HCl | 7.0 - 9.0 |
| HEPES - NaOH | 7.2 - 8.2 |
Additives
Salts
Lysis buffer usually contains one or more salts. The function of salts in lysis buffer is to establish an ionic strength in the buffer solution. Some of the most commonly used salts are NaCl, KCl, and (NH4)2SO4. They are usually used with a concentration between 50 and 150 mM.[4]

Detergent
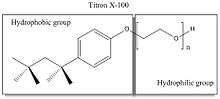
Detergents are organic amphipathic (with hydrophobic tail and a hydrophilic head) surfactants. They are used to separate membrane proteins from membrane because the hydrophobic part of detergent can surround biological membranes and thus isolate membrane proteins from membranes.[5] Although detergents are widely used and have similar functions, it is important to understand the physical and chemical properties of the detergents of interest in order to determine the optimal one to use for your experiment.
Detergents are often categorized as nonionic, anionic, cationic, or zwitterionic, based on their hydrophilic head group feature.[5]
Nonionic detergents like Triton X-100 and zwitterionic detergents like CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) are nondenaturing (will not disrupt protein functions). Ionic detergents like sodium dodecyl sulfate (SDS) and cationic detergents like ethyl trimethyl ammonium bromide are denaturing (will disrupt protein functions).[6] Detergents are a major ingredient that determines the lysis strength of a given lysis buffer.
Others
Other additives include metal ions, sugar like glucose, glycerol, metal chelators (e.g. EDTA), and reducing agents like dithiothreitol (DTT).[4]
Commonly used buffers
NP-40 lysis buffer
It may be the most widely used lysis buffer. The solubilizing agent is NP-40, which can be replaced by other detergents at different concentrations. Since NP-40 is a nonionic detergent, this lysis buffer has a milder effect than RIPA buffer. It can be used when protein functions are to be retained with minimal disruption.[7]
Recipe:[7]
- 150 mM NaCl
- 1.0% Nonidet P-40 or Triton X-100
- 50 mM Tris-Cl
- Adjust pH to 7.4
RIPA (RadioImmunoPrecipitation Assay) lysis buffer
RIPA buffer is a commonly used lysis buffer for immunoprecipitation and general protein extraction from cells and tissues. The buffer can be stored without vanadate at 4 °C for up to 1 year.[8] RIPA buffer releases proteins from cells as well as disrupts most weak interactions between proteins.[7]
Recipe:[8]
- 1% (w/w) Nonidet P-40 (NP-40)
- 1% (w/v) sodium deoxycholate
- 0.1% (w/v) SDS
- 0.15 M NaCl
- 0.01 M sodium phosphate, pH 7.2
- 2 mM EDTA
- 50 mM sodium fluoride (NaF)
- 0.2 mM fresh sodium orthovanadate (Na3VO4.2H2O, it has phosphatase inhibitor function because it mimics phosphate[9])
- 100 U/ml protease inhibitor, such as aprotinin
SDS (sodium dodecyl sulfate) lysis buffer
SDS is ionic denaturing detergent. Hot SDS buffer is often used when the proteins need to be completely solubilized and denatured.
Recipe:[8]
- 0.5% (w/v) SDS
- 0.05 M Tris⋅Cl
- Adjust pH to 8.0
- Add 1 mM fresh dithiothreitol (DTT)
ACK (Ammonium-Chloride-Potassium) lysing buffer
ACK is used for lysis of red blood cells in biological samples where other cells such as white blood cells are of greater interest.[10]
- 150mM ammonium chloride
- 10mM potassium bicarbonate
- 0.1mM EDTA
- Adjust pH to 7.2-7.4
Lysis buffer in DNA and RNA studies
In studies like DNA fingerprinting the lysis buffer is used for DNA isolation. Dish soap can be used in a pinch to break down the cell and nuclear membranes, allowing the DNA to be released. Other such lysis buffers include the proprietary Qiagen product Buffer P2.
References
- Posch, Anton (2014-12-01). "Sample preparation guidelines for two-dimensional electrophoresis". Archives of Physiology and Biochemistry. 120 (5): 192–197. doi:10.3109/13813455.2014.955031. ISSN 1744-4160. PMID 25211021.
- Peach, Mandy; Marsh, Noelle; Miskiewicz, EwaI.; MacPhee, DanielJ. (2015-01-01). Kurien, Biji T.; Scofield, R. Hal (eds.). Solubilization of Proteins: The Importance of Lysis Buffer Choice. Methods in Molecular Biology. 1312. Springer New York. pp. 49–60. doi:10.1007/978-1-4939-2694-7_8. ISBN 9781493926930. PMID 26043989.
- Posch, Anton (2008). 2D PAGE: Sample Preparation and Fractionation. Humana Press. pp. 24. ISBN 978-1-58829-722-8.
- Affairs, EMBL - Office of Information and Public. "Protein Purification - Extraction and Clarification - Choice of lysis buffer and additives - EMBL". www.embl.de. Retrieved 2016-03-16.
- Linke, Dirk (2009-01-01). Deutscher, Richard R. Burgess and Murray P. (ed.). Chapter 34 Detergents: An Overview. Methods in Enzymology. Guide to Protein Purification, 2nd Edition. 463. pp. 603–617. doi:10.1016/s0076-6879(09)63034-2. ISBN 9780123745361. PMID 19892194.
- "Detergents for Cell Lysis and Protein Extraction". www.thermofisher.com. Retrieved 2016-03-16.
- Ji, Hong (2010-08-01). "Lysis of Cultured Cells for Immunoprecipitation". Cold Spring Harbor Protocols. 2010 (8): pdb.prot5466. doi:10.1101/pdb.prot5466. ISSN 1940-3402. PMID 20679375.
- Sefton, Bartholomew M. (2001-01-01). "Labeling Cultured Cells with32Piand Preparing Cell Lysates for Immunoprecipitation". Labeling Cultured Cells with 32Pi and Preparing Cell Lysates for Immunoprecipitation. Current Protocols in Molecular Biology. Chapter 18. John Wiley & Sons, Inc. pp. Unit 18.2. doi:10.1002/0471142727.mb1802s40. ISBN 9780471142720. PMID 18265167.
- "Sample preparation for western blot | Abcam". www.abcam.com. Retrieved 2016-03-16.
- https://www.thermofisher.com/order/catalog/product/A1049201
- "ACK Lysis Buffer". Cold Spring Harbor Protocols. 2014 (11): pdb.rec083295. 2014. doi:10.1101/pdb.rec083295.
- "A10492 - ACK Lysing Buffer - US".