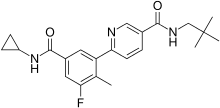
Losmapimod
Losmapimod (GW856553X) is an investigational drug being developed by Fulcrum Therapeutics for the treatment of facioscapulohumeral muscular dystrophy (FSHD) and COVID-19.[1] It selectively inhibits enzymes p38α/β mitogen-activated protein kinases (MAPKs), which are modulators of DUX4 expression and mediators of inflammation.[2]
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.124 |
| Chemical and physical data | |
| Formula | C22H26FN3O2 |
| Molar mass | 383.467 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Historical Investigations
Losmapimod was discovered and unsuccessfully developed by GSK for treating multiple medical conditions. Despite failing to prove efficacy, GSK clinical trials showed that losmapimod is generally well tolerated across more than 3,500 subjects.[3][14][15]
GSK investigated losmapimod as a therapeutic for patients post-myocardial infarction (heart attack). Despite phase II clinical trials[16][17][18] showing improvement of multiple cardiovascular biomarkers,[19][20][21] the phase IIIA clinical trial (LATITUDE)[22] failed to show significantly improved clinical outcomes.[23] In October 2015 GSK announced cancelling the planned phase IIIB trial, but would "evaluate all options for future development."[24]
GSK investigated losmapimod as a therapeutic for COPD, but multiple phase II clinical trials[25][26][27] failed to show that losmapimod improves exercise tolerance,[14] lung function,[14] arterial inflammation,[15] endothelial function,[15] or rate of COPD exacerbations[28] in subjects with COPD. GSK terminated development of losmapimod for COPD in 2016.[29][30]
GSK investigated losmapimod as a therapeutic for major depressive disorder (MDD) on the basis of depression being correlated with elevated pro-inflammatory cytokines.[31] Phase II clinical trials[32][33] failed to show a significant improvement in depression symptoms and biomarkers.[31]
References
- "Losmapimod as potential COVID-19 treatment?". FSHD Society. 10 June 2020. Archived from the original on 10 June 2020. Retrieved 10 June 2020.
- Aston N, Bamborough P, Buckton J, Edwards C, Holmes D, Jones K, Patel V, Smee P, Somers D, Vitulli G, Walker A. p38α Mitogen-Activated Protein Kinase Inhibitors: Optimization of a Series of Biphenylamides to Give a Molecule Suitable for Clinical Progression. Journal of Medicinal Chemistry 2009, 52(20), 6257. doi:10.1021/jm9004779
- "FORM S-1 REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933 FULCRUM THERAPEUTICS, INC". www.sec.gov. Securities and Exchange Commission. Retrieved 26 October 2019.
- Oliva, Jonathan; Galasinski, Scott; Richey, Amelia; Campbell, Amy E.; Meyers, Marvin J.; Modi, Neal; Zhong, Jun Wen; Tawil, Rabi; Tapscott, Stephen J.; Sverdrup, Francis M. (August 2019). "Clinically Advanced p38 Inhibitors Suppress DUX4 Expression in Cellular and Animal Models of Facioscapulohumeral Muscular Dystrophy". Journal of Pharmacology and Experimental Therapeutics. 370 (2): 219–230. doi:10.1124/jpet.119.259663. PMC 6652132. PMID 31189728.
- "In Lab, SLU Research Halts Toxic Protein Linked to Muscular Dystrophy". www.newswise.com. Newswise. 23 October 2019. Retrieved 26 October 2019.
- "Facio to present at the World Muscle Society Congress". Facio Therapies. 30 September 2019. Retrieved 9 November 2019.
- "Facio reveals novel mechanism targeting the cause of FSHD". Facio Therapies. 24 June 2019. Retrieved 9 November 2019.
- Perdiguero, E; Ruiz-Bonilla, V; Gresh, L; Hui, L; Ballestar, E; Sousa-Victor, P; Baeza-Raja, B; Jardí, M; Bosch-Comas, A; Esteller, M; Caelles, C; Serrano, AL; Wagner, EF; Muñoz-Cánoves, P (7 March 2007). "Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation". The EMBO Journal. 26 (5): 1245–56. doi:10.1038/sj.emboj.7601587. PMC 1817635. PMID 17304211.
- Rojas, L. Alejandro; Valentine, Erin; Accorsi, Anthony; Maglio, Joseph; Shen, Ning; Robertson, Alan; Kazmirski, Steven; Rahl, Peter; Tawil, Rabi; Cadavid, Diego; Thompson, Lorin A.; Ronco, Lucienne; Chang, Aaron N.; Cacace, Angela M.; Wallace, Owen (12 July 2019). "P38α Regulates Expression of DUX4 in Facioscapulohumeral Muscular Dystrophy". bioRxiv: 700195. doi:10.1101/700195. Retrieved 9 November 2019.
- Fulcrum Therapeutics Acquires Global Rights to Losmapimod, a Potential Disease-Modifying Therapy for Facioscapulohumeral Muscular Dystrophy
- "Fulcrum Therapeutics Announced Results of Phase 1 Clinical Trial of Losmapimod in FSHD". GlobeNewswire News Room. 4 October 2019.
- Efficacy and Safety of Losmapimod in Subjects With Facioscapulohumeral Muscular Dystrophy (FSHD) (FSHD)
- "Evaluation of Safety, Tolerability, and Changes in Biomarker and Clinical Outcome Assessments of Losmapimod for FSHD1". clinicaltrials.gov. Retrieved 9 October 2019.
- Watz, Henrik; Barnacle, Helen; Hartley, Benjamin F; Chan, Robert (January 2014). "Efficacy and safety of the p38 MAPK inhibitor losmapimod for patients with chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial". The Lancet Respiratory Medicine. 2 (1): 63–72. doi:10.1016/S2213-2600(13)70200-5. PMID 24461903.
- Fisk, M; Cheriyan, J; Mohan, D; Forman, J; Mäki-Petäjä, KM; McEniery, CM; Fuld, J; Rudd, JHF; Hopkinson, NS; Lomas, DA; Cockcroft, JR; Tal-Singer, R; Polkey, MI; Wilkinson, IB (2018). "The p38 mitogen activated protein kinase inhibitor losmapimod in chronic obstructive pulmonary disease patients with systemic inflammation, stratified by fibrinogen: A randomised double-blind placebo-controlled trial". PLOS ONE. 13 (3): e0194197. Bibcode:2018PLoSO..1394197F. doi:10.1371/journal.pone.0194197. PMC 5863984. PMID 29566026.
- "Study To Evaluate The Effects Of GW856553 On Endothelial Function/Vascular Compliance In Subjects With Dyslipidaemia". clinicaltrials.gov. United States National Library of Medicine. Retrieved 12 August 2019.
- "A Study to Evaluate the Effects of 3 Months Dosing With GW856553, as Assessed FDG-PET/CT Imaging". ClinicalTrials.gov. United States National Library of Medicine. Retrieved 12 August 2019.
- A Study to Evaluate the Safety of 12 Weeks of Dosing With GW856553 and Its Effects on Inflammatory Markers, Infarct Size, and Cardiac Function in Subjects With Myocardial Infarction Without ST-segment Elevation (Solstice)
- Cheriyan et al., Circulation 2011, 123(5), 515-523. Inhibition of p38 Mitogen-Activated Protein Kinase Improves Nitric Oxide–Mediated Vasodilatation and Reduces Inflammation in Hypercholesterolemia doi:10.1161/CIRCULATIONAHA.110.971986
- Elkhawad, M; Rudd, JH; Sarov-Blat, L; Cai, G; Wells, R; Davies, LC; Collier, DJ; Marber, MS; Choudhury, RP; Fayad, ZA; Tawakol, A; Gleeson, FV; Lepore, JJ; Davis, B; Willette, RN; Wilkinson, IB; Sprecher, DL; Cheriyan, J (September 2012). "Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis". JACC: Cardiovascular Imaging. 5 (9): 911–22. doi:10.1016/j.jcmg.2012.02.016. PMID 22974804.
- Newby, LK; Marber, MS; Melloni, C; Sarov-Blat, L; Aberle, LH; Aylward, PE; Cai, G; de Winter, RJ; Hamm, CW; Heitner, JF; Kim, R; Lerman, A; Patel, MR; Tanguay, JF; Lepore, JJ; Al-Khalidi, HR; Sprecher, DL; Granger, CB; SOLSTICE, Investigators. (27 September 2014). "Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-ST-segment elevation myocardial infarction: a randomised phase 2 trial". Lancet. 384 (9949): 1187–95. doi:10.1016/S0140-6736(14)60417-7. PMID 24930728.
- "A Phase 3 Clinical Outcomes Study to Compare the Incidence of Major Adverse Cardiovascular Events in Subjects Presenting With Acute Coronary Syndrome Treated With Losmapimod Compared to Placebo (LATITUDE-TIMI 60)". ClinicalTrials.gov. United States National Library of Medicine. Retrieved 12 August 2019.
- O’Donoghue, Michelle L.; Glaser, Ruchira; Cavender, Matthew A.; Aylward, Philip E.; Bonaca, Marc P.; Budaj, Andrzej; Davies, Richard Y.; Dellborg, Mikael; Fox, Keith A. A.; Gutierrez, Jorge Antonio T.; Hamm, Christian; Kiss, Robert G.; Kovar, František; Kuder, Julia F.; Im, Kyung Ah; Lepore, John J.; Lopez-Sendon, Jose L.; Ophuis, Ton Oude; Parkhomenko, Alexandr; Shannon, Jennifer B.; Spinar, Jindrich; Tanguay, Jean-Francois; Ruda, Mikhail; Steg, P. Gabriel; Theroux, Pierre; Wiviott, Stephen D.; Laws, Ian; Sabatine, Marc S.; Morrow, David A. (19 April 2016). "Effect of Losmapimod on Cardiovascular Outcomes in Patients Hospitalized With Acute Myocardial Infarction". JAMA. 315 (15): 1591–9. doi:10.1001/jama.2016.3609. PMID 27043082.
- "GSK provides update on LATITUDE-TIMI 60 (losmapimod cardiovascular study)". GSK. 27 October 2015. Retrieved 12 August 2019.
- "Randomised, Double-Blind, Placebo-Controlled, Parallel-Group, Multi-centre, Dose Ranging Study to Evaluate the Efficacy and Safety of Losmapimod Tablets Administered Twice Daily Compared With Placebo for 24 Weeks in Adult Subjects With Chronic Obstructive Pulmonary Disease (COPD)". ClinicalTrials.gov. United States National Library of Medicine. Retrieved 11 August 2019.
- "Losmapimod in Chronic Obstructive Pulmonary Disease Patients Stratified by Fibrinogen. (EVOLUTION)". ClinicalTrials.gov. United States National Library of Medicine. Retrieved 11 August 2019.
- "Safety and Efficacy Study of Losmapimod (GW856553) in Frequently Exacerbating Participants With Chronic Obstructive Pulmonary Disease (COPD)". ClinicalTrials.gov. United States National Library of Medicine. Retrieved 11 August 2019.
- Pascoe, Steven; Costa, Maria; Marks-Konczalik, Joanna; McKie, Elizabeth; Yang, Shuying; Scherbovsky, Pablo Saez (2017). "Biological effects of p38 MAPK inhibitor losmapimod does not translate to clinical benefits in COPD". Respiratory Medicine. 130: 20–26. doi:10.1016/j.rmed.2017.07.002. ISSN 1532-3064. PMID 29206629.
- Keown, Alex (26 October 2016). "GlaxoSmithKline Terminates Development of Losmapimod for COPD". BioSpace. Retrieved 11 August 2019.
- Lawrence, Stacy (26 October 2016). "GSK drops a pair of late-stage candidates in COPD, HIV". FierceBiotech. Retrieved 11 August 2019.
- Inamdar, Amir; Merlo-Pich, Emilio; Gee, Michelle; Makumi, Clare; Mistry, Prafull; Robertson, Jon; Steinberg, Erik; Zamuner, Stefano; Learned, Susan; Alexander, Robert; Ratti, Emiliangelo (2 April 2014). "Evaluation of antidepressant properties of the p38 MAP kinase inhibitor losmapimod (GW856553) in Major Depressive Disorder: Results from two randomised, placebo-controlled, double-blind, multicentre studies using a Bayesian approach". Journal of Psychopharmacology. 28 (6): 570–581. doi:10.1177/0269881114529377. PMID 24699061.
- "A Study of GW856553X For the Treatment of Depression". ClinicalTrials.gov. United States National Library of Medicine. Retrieved 12 August 2019.
- "Clinical Study to Test a New Drug to Treat Major Depression". ClinicalTrials.gov. United States National Library of Medicine. Retrieved 12 August 2019.