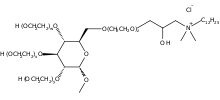
Lauryl methyl gluceth-10 hydroxypropyl dimonium chloride
Lauryl methyl gluceth-10 hydroxypropyl dimonium chloride is an ingredient in some types of soaps and personal care products. It is used as a substantive conditioning humectant.[1][2] This chemical is a type of methyl glucoside derivative,[3] which has been modified by ethoxylation and quaternization.[4] A synthetic pathway for lauryl methyl gluceth-10 hydroxypropyldimonium chloride and other methyl glucoside humectants has been outlined in trade literature.[5]
 | |
| Names | |
|---|---|
| IUPAC name
D-Glucopyranose, methyl ether, ethoxylated, 3-(N-dodecyl-N,N-dimethylammonio)-2-hydroxypropyl ethers (10 mol EO average molar ratio) | |
| Other names
Lauryl methyl gluceth-10-hydroxypropyldimonium chloride, Glucquat 125 | |
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.121.832 |
| EC Number |
|
| Properties | |
| Appearance | Pale, yellow liquid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lauryl methyl gluceth-10 hydroxypropyldimonium chloride is listed as a trade-named raw material, Glucquat 125, in cosmetic and toiletry products.[6]
References
- R. Schueller and P. Romanowski, ed. (1999). Conditioning Agents for Hair and Skin, Cosmetic Science and Technology Series. 21. Marcel Dekker, New York. p. 272.
- A. O. Barel and H. I. Maibach, ed. (Jul 13, 2001). Handbook of Cosmetic Science and Technology. Taylor & Francis.
- "Methyl Glucoside Derivatives". Lubrizol. October 15, 2012. Archived from the original on April 14, 2014. Retrieved April 13, 2014.
- "Glucquat™ 125 Humectant, Technical Data Sheet TDS-546" (PDF). Lubrizol. February 7, 2007.
- "Glucam™ and Glucquat™ Humectants and Emollients" (PDF). Lubrizol. 2009. Archived from the original (PDF) on 2013-03-09. Retrieved 2020-02-24.
- E. W. Flick (1999). Cosmetic and Toiletry Formulations, Second Edition. 7. Noyes Publications/William Andrew Publishing. p. 370.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.