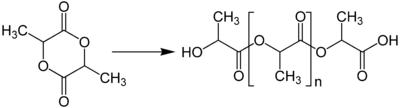
Lactide
Lactide is the lactone cyclic di-ester derived from lactic acid (2-hydroxypropionic acid). With the formula (OCHCO2)2, it exists in three different stereoisomeric forms. All are colorless or white solids. Lactide has attracted great interest because it is derived from abundant renewable resources and is the precursor to a polymer similar to polystyrene, but biodegradable.[3]
 | |
| Names | |
|---|---|
| Other names
Dilactid, (R,R)-3,6-Dimethyl-1,4-dioxan-2,5-dion, (S,S)-3,6-Dimethyl-1,4-dioxan-2,5-dion, (meso)-3,6-Dimethyl-1,4-dioxan-2,5-dion, (R,R)-2,5-Dimethyl-3,6-dioxo-1,4-dioxan, (S,S)-2,5-Dimethyl-3,6-dioxo-1,4-dioxan, (meso)-2,5-Dimethyl-3,6-dioxo-1,4-dioxan | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.245 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H8O4 | |
| Molar mass | 144.126 g·mol−1 |
| Melting point | 95 to 97 °C (203 to 207 °F; 368 to 370 K) [(S,S)-Lactide and (R,R)-Lactide][2] |
| Hydrolyses to lactic acid[2] | |
| Solubility | soluble in chloroform, methanol slightly soluble in benzene |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H319 |
| P264, P280, P305+351+338, P337+313 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Stereoisomers
Lactic acid is chiral such that (R)-lactic acid and (S)-lactic acid exist. Furthermore, these enantiomers do not racemize readily. Thus, formation of lactide from two equivalents of lactic acid gives rise to three stereoisomers:
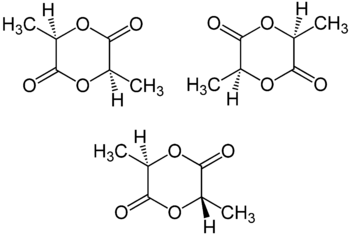
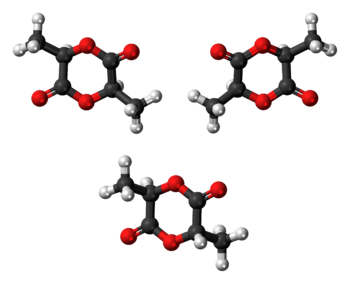
All three stereosiomers undergo epimerisation in the presence of organic and inorganic bases in solution.[4]
Polymerization
Lactide can be polymerized to polylactic acid (polylactide). Depending on the catalyst, syndiotactic or a heterotactic polymers can result. The resulting materials, polylactic acid, have many attractive properties.[5][6]
References
- Sigma Aldrich product page for lactide Retrieved 8th of July 2015
- Römpp Online Chemielexikon Version 3.3 aufgerufen am 25. März 2009
- Andreas Künkel, Johannes Becker, Lars Börger, Jens Hamprecht, Sebastian Koltzenburg, Robert Loos, Michael Bernhard Schick, Katharina Schlegel, Carsten Sinkel, Gabriel Skupin and Motonori Yamamoto (2016). "Polymers, Biodegradable". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–29. doi:10.1002/14356007.n21_n01.pub2. ISBN 9783527306732.CS1 maint: uses authors parameter (link)
- Shuklov, Ivan A.; Jiao, Haijun; Schulze, Joachim; Tietz, Wolfgang; Kühlein, Klaus; Börner, Armin (2011-03-02). "Studies on the epimerization of diastereomeric lactides". Tetrahedron Letters. 52 (9): 1027–1030. doi:10.1016/j.tetlet.2010.12.094. ISSN 0040-4039.
- R. Auras; L.-T. Lim; S. E. M. Selke; H. Tsuji (2010). Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications. Wiley. ISBN 978-0-470-29366-9.
- Odile Dechy-Cabaret, Blanca Martin-Vaca and Didier Bourissou (2004). "Controlled Ring-Opening Polymerization of Lactide and Glycolide". Chem. Rev. 104 (12): 6147–76. doi:10.1021/cr040002s. PMID 15584698.CS1 maint: uses authors parameter (link)