LACTB
Serine beta-lactamase-like protein LACTB, mitochondrial is an enzyme that in humans is encoded by the LACTB gene.[5][6] This gene encodes a 54 kDa protein sharing significant sequence similarity to serine proteases of the penicillin binding protein and beta-lactamase superfamily occurring in bacteria. [7] It is involved in the regulation of the metabolic circuitry. A causal association has been found between LACTB and obesity.[8] In breast cancer, LACTB has a tumor suppressor function by modulating lipid metabolism.[9]
| LACTB | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||
| Aliases | LACTB, G24, MRPL56, lactamase beta | ||||||||||||||||||||||||
| External IDs | OMIM: 608440 MGI: 1933395 HomoloGene: 12755 GeneCards: LACTB | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||||||||||
| RefSeq (protein) | |||||||||||||||||||||||||
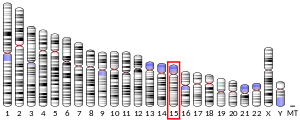
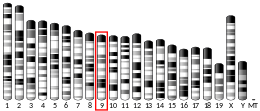
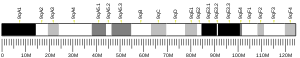
| Location (UCSC) | Chr 15: 63.12 – 63.14 Mb | Chr 9: 66.96 – 66.98 Mb | |||||||||||||||||||||||
| PubMed search | [3] | [4] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Structure
Gene
The LACTB gene is located at chromosome 15q22.1, consisting of 8 exons. Alternative splicing results in multiple transcript variants encoding different protein isoforms.
Protein
LACTB shares sequence similarity to the beta-lactamase/penicillin-binding protein family of serine proteases that are involved in bacterial cell wall metabolism. The N-terminal 97 amino acid segment of LACTB does not form part of the conserved penicillin-binding protein domain and may therefore be responsible for organelle targeting.[7][10]
Function
LACTB is widely expressed in different mammalian tissues, with the predominant expression in human skeletal muscle. It localizes in the mitochondrial intermembrane space.[10] LACTB can polymerize into stable filaments occupying the mitochondrial intermembrane space. These filaments are speculated to play a role in submitochondrial organization and therefore possibly affect mitochondrial metabolon organization.[10]
Clinical significance
It has been found LACTB could cause obesity through gene co-expression analysis based on data integrated from multiple sources. This has been validated in vivo through LACTB overexpression in transgenic mice, which resulted in an obese phenotype.[8] LACTB has also been identified to be a tumor suppressor through its effect on mitochondrial phospholipid metabolism and modulation of cell differentiation state.[11]
Interactions
- MiR-125b-5p [12]
References
- GRCh38: Ensembl release 89: ENSG00000103642 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000032370 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Smith TS, Southan C, Ellington K, Campbell D, Tew DG, Debouck C (November 2001). "Identification, genomic organization, and mRNA expression of LACTB, encoding a serine beta-lactamase-like protein with an amino-terminal transmembrane domain". Genomics. 78 (1–2): 12–4. doi:10.1006/geno.2001.6643. PMID 11707067.
- "Entrez Gene: LACTB lactamase, beta".
- Peitsaro N, Polianskyte Z, Tuimala J, Pörn-Ares I, Liobikas J, Speer O, Lindholm D, Thompson J, Eriksson O (2008). "Evolution of a family of metazoan active-site-serine enzymes from penicillin-binding proteins: a novel facet of the bacterial legacy". BMC Evolutionary Biology. 8: 26. doi:10.1186/1471-2148-8-26. PMC 2266909. PMID 18226203.
- Chen Y, Zhu J, Lum PY, Yang X, Pinto S, MacNeil DJ, Zhang C, Lamb J, Edwards S, Sieberts SK, Leonardson A, Castellini LW, Wang S, Champy MF, Zhang B, Emilsson V, Doss S, Ghazalpour A, Horvath S, Drake TA, Lusis AJ, Schadt EE (March 2008). "Variations in DNA elucidate molecular networks that cause disease". Nature. 452 (7186): 429–35. doi:10.1038/nature06757. PMC 2841398. PMID 18344982.
- Eriksson, Ove; Lalowski, Maciej; Lindholm, Dan (2017). "Commentary: LACTB is a tumour suppressor that modulates lipid metabolism and cell state". Frontiers in Physiology. 8: 396. doi:10.3389/fphys.2017.00396. ISSN 1664-042X. PMC 5462942. PMID 28642719.
- Polianskyte Z, Peitsaro N, Dapkunas A, Liobikas J, Soliymani R, Lalowski M, Speer O, Seitsonen J, Butcher S, Cereghetti GM, Linder MD, Merckel M, Thompson J, Eriksson O (November 2009). "LACTB is a filament-forming protein localized in mitochondria". Proceedings of the National Academy of Sciences of the United States of America. 106 (45): 18960–5. doi:10.1073/pnas.0906734106. PMC 2767363. PMID 19858488.
- Keckesova et al. 2017. LACTB is a tumour suppressor that modulates lipid metabolism and cell state. Nature 543:681-686
- Lu JB, Yao XX, Xiu JC, Hu YW (January 2016). "MicroRNA-125b-5p attenuates lipopolysaccharide-induced monocyte chemoattractant protein-1 production by targeting inhibiting LACTB in THP-1 macrophages". Archives of Biochemistry and Biophysics. 590: 64–71. doi:10.1016/j.abb.2015.11.007. PMID 26603571.
Further reading
- Hallis TM, Kopp AL, Gibson J, Lebakken CS, Hancock M, Van Den Heuvel-Kramer K, Turek-Etienne T (August 2007). "An improved beta-lactamase reporter assay: multiplexing with a cytotoxicity readout for enhanced accuracy of hit identification". Journal of Biomolecular Screening. 12 (5): 635–44. doi:10.1177/1087057107301499. PMID 17517902.
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (November 2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
- Clark HF, Gurney AL, Abaya E, Baker K, Baldwin D, Brush J, Chen J, Chow B, Chui C, Crowley C, Currell B, Deuel B, Dowd P, Eaton D, Foster J, Grimaldi C, Gu Q, Hass PE, Heldens S, Huang A, Kim HS, Klimowski L, Jin Y, Johnson S, Lee J, Lewis L, Liao D, Mark M, Robbie E, Sanchez C, Schoenfeld J, Seshagiri S, Simmons L, Singh J, Smith V, Stinson J, Vagts A, Vandlen R, Watanabe C, Wieand D, Woods K, Xie MH, Yansura D, Yi S, Yu G, Yuan J, Zhang M, Zhang Z, Goddard A, Wood WI, Godowski P, Gray A (October 2003). "The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment". Genome Research. 13 (10): 2265–70. doi:10.1101/gr.1293003. PMC 403697. PMID 12975309.
- Hell-Pourmojib M, Neuner P, Fischer H, Rezaie S, Kindås-Mügge I, Knobler R, Trautinger F (July 2002). "Differential expression of a novel gene in response to hsp27 and cell differentiation in human keratinocytes". The Journal of Investigative Dermatology. 119 (1): 154–9. doi:10.1046/j.1523-1747.2002.01793.x. PMID 12164938.
- Koc EC, Burkhart W, Blackburn K, Moyer MB, Schlatzer DM, Moseley A, Spremulli LL (November 2001). "The large subunit of the mammalian mitochondrial ribosome. Analysis of the complement of ribosomal proteins present". The Journal of Biological Chemistry. 276 (47): 43958–69. doi:10.1074/jbc.M106510200. PMID 11551941.