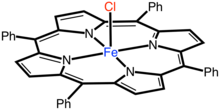
Iron(tetraphenylporphyrinato) chloride
Iron(tetraporphyriinato) chloride is the coordination complex with the formula Fe(TPP)Cl where TPP is the dianion [C44H28N4]2-. The compound is a blue microcrystals that dissolve in chlorinated solvent to give brown solutions. In terms of structure, the complex is five-coordinate with idealized C4v point group symmetry.[1]
 | |
| Names | |
|---|---|
| Other names
fecl(tpp) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C44H28ClFeN4 | |
| Molar mass | 704.03 g·mol−1 |
| Appearance | dark blue solid |
| Density | 1.318 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions
Fe(TPP)Cl is prepared by the reaction of tetraphenylporphyrin (H2TPP) and ferrous chloride in the presence of air:
- H2TPP + FeCl2 + 1/4 O2 → Fe(TPP)Cl + HCl + 1/2 H2O
The chloride can be replaced with other halides and pseudohalides. Base gives the "mu-oxo dimer":
- 2 Fe(TPP)Cl + 2 NaOH → [Fe(TPP)]2O + 2 NaCl + H2O
Most relevant to catalysis, the complex is easily reduced to give ferrous derivatives (L = pyridine, imidazole):
- Fe(TPP)Cl + e- + 2 L → Fe(TPP)L2 + Cl-
References
- Hunter, Seth C.; Smith, Brenda A.; Hoffmann, Christina M.; Wang, Xiaoping; Chen, Yu-Sheng; McIntyre, Garry J.; Xue, Zi-Ling (2014). "Intermolecular Interactions in Solid-State Metalloporphyrins and Their Impacts on Crystal and Molecular Structures". Inorganic Chemistry. 53 (21): 11552–11562. doi:10.1021/ic5015835. PMID 25338536.
- Cui, Xin; Zhang, X. Peter (2012). "Iron(III)meso-Tetraphenylporphine Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn01388. ISBN 978-0471936237.
- Pegis, M. L.; Martin, D. J.; Wise, C. F.; Brezny, A. C.; Johnson, S. I.; Johnson, L. E.; Kumar, N.; Raugei, S.; Mayer, J. M. (2019). "Mechanism of Catalytic O2 Reduction by Iron Tetraphenylporphyrin". Journal of the American Chemical Society. 141 (20): 8315–8326. doi:10.1021/jacs.9b02640. PMC 6684231. PMID 31042028.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.