Intravitreal administration
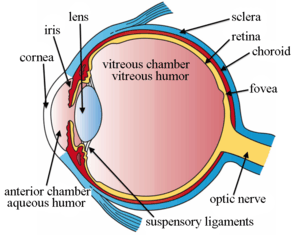
Intravitreal is a route of administration of a drug, or other substance, in which the substance is delivered into the vitreous humor of the eye. "Intravitreal" literally means "inside an eye". Intravitreal injections were first introduced in 1911 when Ohm gave an injection of air into the vitreous humor to repair a detached retina. In the mid-1940s, intravitreal injections became a standard way to administer drugs to treat endophthalmitis and cytomegalovirus retinitis.[1]
Epidemiology
Intravitreal injections were proposed over a century ago however the number performed remained relatively low until the mid 2000s. Until 2001, intravitreal injections were mainly used to treat end-ophthalmitis. The number of intravitreal injections stayed fairly constant, around 4,500 injections per year in the US.[2] The number of injections tripled to 15,000 in 2002 when triamcinolone injections were first used to treat diabetic macular oedema.[2][3] This use continued to drive an increase to 83,000 injections in 2004.[2] In 2005 bevacizumab and ranibizumab intravitreal injections for the treatment of wet-AMD caused a rise in injections to 252,000.[2] In 2008, over 1 million intravitreal injections were performed. This doubled to 2 million just 3 years later in 2011 when another anti-VEGF intravitreal injection aflibercept became available for the treatment of wet AMD.[2] Intravitreal injections hit an all time high in 2016 reaching over 5.9 million injections in the US.[1]
Uses
Anti-Vascular Endothelial Growth Factor (anti-VEGF)
The most common reason intravitreal injections are used is to administer anti-vascular endothelial growth factor (anti-VEGF) therapies to treat wet age related macular degeneration (AMD) and diabetic retinopathy. Both of these conditions cause damage to the retina leading to vision loss. There are three widely used Anti-VEGF drugs to treat these conditions: ranibizumab (Lucentis®; Genentech), bevacizumab (Avastin®; Genentech), and aflibercept (Eylea®; Regeneron Pharmaceuticals). Bevacizumab has not been FDA approved to treat wet AMD however in the US it is the first line anti-VEGF therapy for over half of ophthalmologists due to its efficacy and drastically lower cost.[4] These three drugs bind to VEGF molecules preventing them from binding to VEGF receptors on the surface of endothelial cells thereby stopping the abnormal angiogenesis that causes wet AMD. All three of these therapies have vastly improved outcomes for sufferers who had limited treatment options prior to their invention but must be administerd via intravitreal injection.
Steroids
Steroids may be administered via intravitreal injection to treat diabetic and vasculo-occlusive macular edema, exudative macular degeneration, pseudophakic cystoid macular edema, and posterior uveitis. Common steroids used to treat these conditions include dexamethasone and triamcinolone acetonide (Triescence, Alcon Laboratories, Inc.). Steroid implants, such as the dexamethasone implant (Ozurdex, Allergan, Inc.), are used for long term treatment of macular edema. Both of these steroid work by modulating inflammatory cytokines.[5]
Adverse Events and Complications
Endophthalmitis
Endophthalmitis is one of the most severe complications due to intravitreal injections. Incidence of endophthalmitis after intravitreal injection per patient has been reported to range from 0.019 to 1.6% these rates are similar across injection settings and geographic locations.[6] Endophthalmitis is an bacterial infection within the eye which causes the white of the eye to be inflamed, white or yellow discharge inside the eyelid, and a white clouded cornea. A layer of white cells called hypopyon may develop between the iris and the cornea. Endophthalmitis is incredibly dangerous and treatment is often an emergency. Injections of antibiotics and antifungal compounds are used to treat endophthalmitis. In more severe cases a vitrectomy may be required to surgically remove infectious debris.[7]
Intraocular Inflammation
Intraocular inflammation is one of the main causes of temporary pain and vision loss after an intravitreal injection. Severe inflammation can cause damage to the eye. Rate of inflammation is dependent upon the drug administered via intravitreal injections. A large clinical trial of ranibizumab for AMD reported intraocular inflammation rates between 1.4–2.9%. Clinical trials of a similar drug administered via intravitreal injection, bevacizumab, reported a much lower incidence ranging from 0.09-0.4%.[6]
Retinal Detachment
Rhegmatogenous retinal detachment after intravitreal injection is low between 0 to 0.67%.[6] A rhegmatogenous retinal detachment is basically when there is a retinal break that allows fluid from the vitreous chamber into the subretinal space. This fluid causes sensory tissues to pull away from the retina and their nutrition source slowly killing the cells.[8]
Ocular Hemorrhage
Subconjunctival hemorrhage is the most common type of hemorrhage following intravitreal injection with a reported incidence of nearly 10% of injections. Aspirin may increase these chances. Massive choroidal hemorrhage and massive subretinal hemorrhage are uncommon but have been reported.[6]
Repeated Injections
Treatments administered via intravitreal injection are not cures and therefore repeated injections are necessary for managing conditions. For example anti-VEGF therapies must be injected monthly or bi-monthly for the rest of their lives in order to treat wet age related macular degeneration. A growing body of evidence has shown repeat intravitreal injections have their own increased risks and complications.
A 3x rise in intraocular pressure after an intravitreal injection is expected and usually only lasts a few minutes.[9] Studies have shown an increased risk of sustained elevated intraocular pressure due to repeated intravitreal injections.[6] Elevated intraocular pressure leads to tissue damage, this is how glaucoma damages the eye. Many theories as to why this is have been postulated however many focus on the effect of the repeated eye trauma. The risk of elevated intraocular pressure is so great that it is recommended clinicians monitor intraocular pressure before and after intravitreal injection.[10] Mount Sinai researchers have developed a method to measure retina damage from long term intravitreal injection using optimal coherence tomography angiography (OCTA). OCTA captures the motion of red blood cells in blood vessels noninvasively allowing researchers to measure blood flow in the macula and optic nerve. From this data they were able to show areas of cumulative damage.
Procedure and Guidelines
In 2004 with the rise of intravitreal injections, a group of experts established the first general guidelines for administering intravitreal injections. Until an update in 2014 these were consensus guidelines in the US. In 2014 a panel of 16 health professionals with expertise in different aspects of the injection reviewed and revised the original guidelines. Together they released areas of general agreement, areas with no clear consensus, and recommended sequence of steps for intravitreal injection.[10]
Recommended Sequence of Steps for IVT Injection (2014)*
- Take a procedural time-out to verify patient, agent and laterality;
- Apply liquid anesthetic drops to the ocular surface;
- Apply povidone-iodine to the eyelashes and eyelid margins (optional, most use 10%);
- Retract the eyelids away from the intended injection site for the duration of the procedure;
- Apply povidone-iodine to the conjunctival surface, including the intended injection site (most use 5%);
- If additional anesthetic is applied, reapply povidone-iodine to the intended injection site immediately prior to injection (most use 5%);
- Insert the needle perpendicular to the sclera, 3.5 to 4 mm posterior to the limbus, between the vertical and horizontal rectus muscles. Application of a sterile cotton-tip applicator over the injection site immediately following removal of the needle may reduce vitreous reflux.
Areas of General Agreement by Committee Members (2014)*
- Povidone-iodine (5-10%) should be the last agent applied to the intended injection site before injection. If a gel anesthetic is used, povidone-iodine should be applied both before and after application of gel, as retained gel may prevent povidone-iodine from contacting the conjunctival surface of the injection site.
- Pre-, peri- or post-injection topical antibiotics are unnecessary.
- There is no evidence to support the routine use of a sterile drape.
- Avoid contamination of the needle and injection site by the eyelashes or the eyelid margins.
- Avoid extensive massage of the eyelids either pre- or post-injection (to avoid meibomian gland expression).
- Use adequate anesthetic for a given patient (topical drops, gel and/or subconjunctival injection).
- Use of sterile or nonsterile gloves as consistent with modern office practice, combined with strong agreement regarding the need for handwashing before and after patient contact.
- Either surgical masks should be used or both the patient and providers should minimize speaking during the injection preparation and procedure to limit aerosolized droplets containing oral contaminants from the patient and/or provider.
- Monitor IOP both pre- and post-injection.
- Routine anterior chamber paracentesis is not recommended.
Areas with No Clear Consensus by Committee Members (2014)*
- Need for povidone-iodine application to the eyelids, including the eyelashes and eyelid margins. All agreed that when povidone-iodine is applied to the eyelashes and eyelid margins, eyelid scrubbing or eyelid pressure adequate to express material from the meibomian glands should be avoided.
- Use of an eyelid speculum (some prevent contact between the needle/injection site and the eyelashes and eyelids with manual lid retraction).
- Need for pupillary dilation and post-injection dilated examination of the posterior segment (although some viewed the return of formed vision as sufficient, others routinely dilate the pupil and examine the posterior segment after injection).
- Use of povidone-iodine flush (most preferred drops only and saw no benefit to allowing the povidone-iodine to dry before injection).
* Reproduced from Avery RL, Bakri SJ, Blumenkranz MS, et al. Retina 2014 Dec; 34 Suppl 12:S1-S18.[10]
Changes from 2004 Guidance
Dropped Recommendations from 2004
Use of a lid speculum is no longer essential. Now a lid speculum, manual lid retraction or a similar maneuver can be used to keep the eyelids out of the way during the procedure.
The strong 2004 consensus that the pupil should be routinely dilated to examine the posterior segment of the eye post injection was dropped. Some of the 2014 panelists did not dilate the pupil for routine injections while others found this examination to be highly important. As no consensus was reached this recommendation was dropped from the 2014 guidance.
New Recommendations in 2014
In 2004 the committee did not come to a consensus on routine use of pre-, peri- or postinjection antibiotics. Since then evidence has emerged suggesting that peri-injection antibiotics do not meaningfully lower the risk of post-injection infection and periodic multi-day administration of topical ophthalmic antibiotics facilitates the colonization of drug-resistant bacteria.[11][12][13][14][15][16] For these reasons in 2014 the committee decided against recommending routine antibiotics.
The new guidelines include hand washing and glove use consistent with the modern-day medical practice of universal precautions. Although the use of gloves was agreed upon by the committee some panelists cited studies showing no impact of glove use on endophthalmitis rate.[11][14]
In 2004, the topic of droplet contamination was not addressed. Since then new evidence has come to light showing that streptococcal species cause a disproportionate number of post intravitreal injection endophthalmitis cases compared to other forms of ocular surgery.[17][18] This is likely due to aerosolized droplet contamination from either the practitioners’ or patients’ mouth.[19] The 2014 guidelines were updated to address these findings recommending both clinicians and patients wear face masks during the procedure.
The new guidelines recommend monitoring intraocular pressure both pre- and post-injection. This recommendation stemmed from new evidence showing that routine intravitreal administration of anti-VEGF therapies may increase intraocular pressure for a sustained time period.[20]
The 2014 guidelines addressed bilateral injections done in the same visit. The committee recommended treating each eye as a separate procedure and use different lots or batches of medication whenever possible. The panel was not able to support the use of sterile drapes in the procedure as retrospective studies showed no increased rate of endophthalmitis in injections done without drapes.[21]
Potential Alternatives
Intravitreal injections have vastly improved outcomes for patients with retinal diseases however the risk and patient burden associated with repeated injections has prompted researchers to pursue less invasive methods of application. There has been significant emphasis on finding methods to administer treatments topically over the last 50 years.[22] This research has garnered more attention thanks to the increase in intravitreal injections and the growing evidence linking repeat injections to adverse events.
See also
- Eye drops
References
- Grzybowski, Andrzej; Told, Reinhard; Sacu, Stefan; Bandello, Francesco; Moisseiev, Elad; Loewenstein, Anat; Schmidt-Erfurth, Ursula (May 2018). "2018 Update on Intravitreal Injections: Euretina Expert Consensus Recommendations". Ophthalmologica. Ophthalmologica 239 (4). 239 (4): 181–193. doi:10.1159/000486145. PMID 29393226. Retrieved 2020-05-02.
- Mich, George A. Williams, MD, Royal Oak. "IVT Injections: Health Policy Implications". www.reviewofophthalmology.com. Retrieved 2020-05-02.
- "The Use of Intravitreal Triamcinolone Acetonide – An Overview". touchOPHTHALMOLOGY. 2011-01-25. Retrieved 2020-05-02.
- "Comparison of Anti-VEGF Treatments for Wet AMD". American Academy of Ophthalmology. 2020-02-03. Retrieved 2020-05-03.
- Sides Media, www sidesmedia com. "Retina Today - Use of Intravitreal Steroids in the Clinic". Retina Today. Retrieved 2020-05-03.
- Ghasemi Falavarjani, K; Nguyen, Q D (July 2013). "Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature". Eye. 27 (7): 787–794. doi:10.1038/eye.2013.107. ISSN 0950-222X. PMC 3709385. PMID 23722722.
- "Endophthalmitis - The American Society of Retina Specialists". www.asrs.org. Retrieved 2020-05-02.
- "Retinal Detachment: The Three Types". WebMD. Retrieved 2020-05-02.
- "Breakthrough Technology Used to Discover Eye Damage from Repeated Intravitreal Injections | Mount Sinai - New York". Mount Sinai Health System. Retrieved 2020-05-02.
- MPH, Colin A. McCannel, MD, Harry W. Flynn, Jr , MD, and Emmett T. Cunningham Jr , MD, PhD. "Updated Guidelines for Intravitreal Injection". www.reviewofophthalmology.com. Retrieved 2020-05-02.
- Bhavsar, Abdhish R. (2009-12-14). "Risk of Endophthalmitis After Intravitreal Drug Injection When Topical Antibiotics Are Not Required". Archives of Ophthalmology. 127 (12): 1581–3. doi:10.1001/archophthalmol.2009.304. ISSN 0003-9950. PMC 2874253. PMID 20008710.
- Stockdale, Cynthia R. (2012-06-01). "Update on Risk of Endophthalmitis After Intravitreal Drug Injections and Potential Impact of Elimination of Topical Antibiotics". Archives of Ophthalmology. 130 (6): 809–10. doi:10.1001/archophthalmol.2012.227. ISSN 0003-9950. PMC 3489025. PMID 22801859.
- Storey, Philip; Dollin, Michael; Pitcher, John; Reddy, Sahitya; Vojtko, Joseph; Vander, James; Hsu, Jason; Garg, Sunir J. (January 2014). "The Role of Topical Antibiotic Prophylaxis to Prevent Endophthalmitis after Intravitreal Injection". Ophthalmology. 121 (1): 283–289. doi:10.1016/j.ophtha.2013.08.037. ISSN 0161-6420. PMID 24144453.
- Cheung, Crystal S.Y.; Wong, Amanda W.T.; Lui, Alex; Kertes, Peter J.; Devenyi, Robert G.; Lam, Wai-Ching (August 2012). "Incidence of Endophthalmitis and Use of Antibiotic Prophylaxis after Intravitreal Injections". Ophthalmology. 119 (8): 1609–1614. doi:10.1016/j.ophtha.2012.02.014. ISSN 0161-6420. PMID 22480743.
- Bhatt, Shabari S; Stepien, Kimberly E; Joshi, Komal (November 2011). "Prophylactic Antibiotic Use After Intravitreal Injection". Retina. 31 (10): 2032–2036. doi:10.1097/iae.0b013e31820f4b4f. ISSN 0275-004X. PMC 4459136. PMID 21659941.
- Dave, Sarita B.; Toma, Hassanain S.; Kim, Stephen J. (October 2011). "Ophthalmic Antibiotic Use and Multidrug-Resistant Staphylococcus epidermidis". Ophthalmology. 118 (10): 2035–2040. doi:10.1016/j.ophtha.2011.03.017. ISSN 0161-6420. PMID 21856006.
- Mccannel, Colin A (April 2011). "Meta-Analysis of Endophthalmitis After Intravitreal Injection of Anti–Vascular Endothelial Growth Factor Agents". Retina. 31 (4): 654–661. doi:10.1097/iae.0b013e31820a67e4. ISSN 0275-004X. PMID 21330939.
- Chen, Eric; Lin, Michael Y; Cox, Joel; Brown, David M (September 2011). "ENDOPHTHALMITIS AFTER INTRAVITREAL INJECTION: The Importance of Viridans Streptococci". Retina. 31 (8): 1525–1533. doi:10.1097/IAE.0b013e318221594a. ISSN 0275-004X. PMID 21878800.
- Wen, Joanne C. (2011-12-01). "Bacterial Dispersal Associated With Speech in the Setting of Intravitreous Injections". Archives of Ophthalmology. 129 (12): 1551. doi:10.1001/archophthalmol.2011.227. ISSN 0003-9950. PMID 21825179.
- Choi, Daniel Y; Ortube, Maria Carolina; Mccannel, Colin A; Sarraf, David; Hubschman, Jean-Pierre; Mccannel, Tara A; Gorin, Michael B (June 2011). "Sustained Elevated Intraocular Pressures After Intravitreal Injection of Bevacizumab, Ranibizumab, and Pegaptanib". Retina. 31 (6): 1028–1035. doi:10.1097/IAE.0b013e318217ffde. ISSN 0275-004X. PMID 21836409.
- Pilli, Suman; Kotsolis, Athanasios; Spaide, Richard F.; Slakter, Jason; Freund, K. Bailey; Sorenson, John; Klancnik, James; Cooney, Michael (May 2008). "Endophthalmitis Associated with Intravitreal Anti-Vascular Endothelial Growth Factor Therapy Injections in An Office Setting". American Journal of Ophthalmology. 145 (5): 879–882. doi:10.1016/j.ajo.2007.12.036. ISSN 0002-9394. PMID 18329624.
- "Home Organization Selection". docs.shib.ncsu.edu. doi:10.1089/jop.2015.0047. PMID 26666398. Retrieved 2020-05-03.