Immunocore
Immunocore is a privately owned British clinical-stage biotechnology company,[3] based in Oxfordshire, which researches and develops biological drugs to treat cancer, infectious diseases and autoimmune diseases using soluble T-cell receptor (TCR) technology.
 | |
| Private | |
| Industry | Biotechnology |
| Founded | 2008[1] in Oxford, England |
| Founders | Dr. Bent Jakobsen and James Noble[2] |
| Headquarters | , |
Key people | Andrew Hotchkiss (CEO) Jonathan Knowles (Chairman) Nicholas Cross (Deputy Chairman of the Board) Eva-Lotta Allan (Chief Business Officer) |
| Products | Cancer drugs/treatments using T-Cell receptor technology |
| Website | www |
History
Immunocore was founded in 2008 as a spinout of German Medigene AG who had acquired former Avidex in 2006 (founded in 1999 as a spinout from the University of Oxford by Dr Bent Jakobsen) and CEO James Noble [3]
In July 2013, GlaxoSmithKline paid ₤142 million for the rights to pre-clinical drugs they had developed which act against several targets that Immunocore had been working on.[4] GlaxoSmithKline has to date selected three targets as part of this discovery collaboration. The lead programme with GSK is on track for an Investigational New Drug (IND) application in 2017 with potential application in Non-Small Cell Lung Cancer (NSCLC), bladder cancer, synocial sarcoma, melanoma and ovarian cancer.[5] In June 2013, Immunocore entered into a similar deal with Genentech, which, according to the deal, would earn Immunocore an initiation fee of between $10 million and $20 million for each lead programme initiated by Genentech and in excess of $300 million in milestone payments for each target programme and significant tiered royalties.[6] In January 2014, MedImmune - the global biologics research and development arm of AstraZeneca announced the next oncology research collaboration and licensing agreement for Immunocore; an upfront payment of $20 million and up to $300 million in development and commercial milestone payments for each target programme selected as well as significant tiered royalties if these programmes were successful.
In July 2015, Immunocore announced the completion of a $320 million private financing round.[7] Fidelity Management & Research Company, Woodford Investment Management, Malin Corporation, Eli Lilly and Company, and RTW Investments all participated in this fundraise along with other unnamed investors and existing shareholders.
In September 2017, the Bill & Melinda Gates Foundation announced that they would invest up to $40 million to support the development of Immunocore's soluble TCR-based therapeutics for infectious diseases.[8] The collaboration was set up to discover and develop ImmTAV® (Immune mobilising monoclonal TCRs Against Virus) and ImmTAB™ (Immune mobilising monoclonal TCRs Against Bacteria) molecules for the treatment of tuberculosis (TB) and human immunodeficiency virus (HIV) [9] where the TCR-based therapeutics have the potential to reduce treatment timelines and improve patient outcomes. This partnership is part of a larger initiative within Immunocore to apply its soluble TCR-based therapeutics to areas outside of oncology, including infectious diseases and autoimmune diseases.[10]
Scientific background
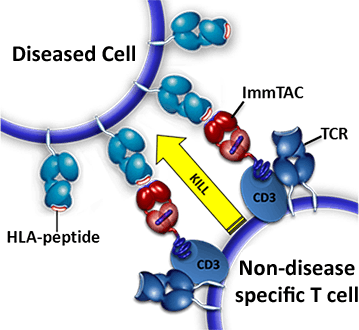
TCRs are molecules found on the surface of T lymphocytes (or T cells) and play various roles in the immune system. TCRs are often cited as aiding in recognising foreign antigens being presented by cells which have been infected by viruses or intracellular bacteria. Immunocore has developed a line of biologic medicines by combining engineered, cancer-recognising, soluble TCRs with immune activating complexes, which, in theory, creates a drug that directs the immune system to kill cancer cells. These drugs are commonly referred to as immune-mobilising monoclonal TCRs against cancer (ImmTAC molecules).[11]
Immunocore's TCR technology has a broad applicability to a wide range of intracellular targets and disease indications including solid tumours, infectious diseases and autoimmune diseases.[12]
Clinical pipeline
IMCgp100
Immunocore's wholly owned lead programme, IMCgp100,[13] is an immunotherapy for metastatic uveal and cutaneous melanoma and is currently in a pivotal clinical study as a monotherapy for the treatment of patients with metastatic uveal melanomas.[14] Immunocore has also entered into combination trials with IMCgp100 in metastatic cutaneous melanoma with MedImmune (AstraZeneca)[15] and in metastatic uveal melanoma with Lilly.[16]
IMCgp100 was granted orphan drug designation for the treatment of uveal melanoma by the US Food and Drug Administration (FDA) in 2016.
Corporate governance
The inaugural chairman of Immunocore's board of directors was Nicholas Cross.[17] In late 2013, Jonathan Knowles succeeded Cross in this position, and Cross was retained as deputy chairman.[17] Knowles had previously served as a non-executive director since 2010.[17]
Eliot Forster served as chief executive officer (CEO)[18] of Immunocore from February 2015 to February 2018. The temporary CEO was Andrew Hotchkiss,[19] who previously served as Chief Commercial Officer (CCO) at Immunocore. The current CEO is Bahija Jallal.
References
- "Transformational Science that Transforms Lives - Immunocore". www.immunocore.com. Retrieved 10 November 2017.
- "Transformational Science that Transforms Lives - Immunocore". www.immunocore.com. Retrieved 10 November 2017.
- "Transformational Science that Transforms Lives - Immunocore". www.immunocore.com. Retrieved 10 November 2017.
- "GSK taps UK firm for "beyond antibodies" cancer drug technology". Fox News Channel. 9 July 2013. Retrieved 9 July 2013.
- "Immunocore Announces Third Oncology Target Selected in Discovery Collaboration with GlaxoSmithKline | News - Immunocore". www.immunocore.com. Retrieved 10 November 2017.
- "Genentech Makes Potentially $300M+ Pact for Immunocore's ImmTACs | GEN". GEN. Retrieved 10 November 2017.
- Halpin, Padraic. "Immunocore raises $320 million in record financing round". U.K. Retrieved 10 November 2017.
- andrewvolkoff (18 September 2017). "Gates Foundation invests $40 million in Immunocore's efforts". GMP news. Retrieved 10 November 2017.
- "British Unicorn Gets $40M Boost from Bill & Melinda Gates Foundation". Labiotech.eu. 18 September 2017. Retrieved 10 November 2017.
- "Gates Foundation supports development of Immunocore's therapeutics for infectious diseases - Pharmaceutical Technology". Pharmaceutical Technology. 18 September 2017. Retrieved 10 November 2017.
- Liddy, Nathaniel; Bossi, Giovanna; Adams, Katherine J; Lissina, Anna; Mahon, Tara M; Hassan, Namir J; Gavarret, Jessie; Bianchi, Frayne C; Pumphrey, Nicholas J (6 May 2012). "Monoclonal TCR-redirected tumor cell killing". Nature Medicine. 18 (6): 980–987. doi:10.1038/nm.2764. ISSN 1546-170X. PMID 22561687.
- "Targets - Immunocore". www.immunocore.com. Retrieved 10 November 2017.
- "Study to Assess the Tolerability of a Bispecific Targeted Biologic IMCgp100 in Malignant Melanoma - Full Text View - ClinicalTrials.gov". Retrieved 10 November 2017.
- GmbH, finanzen.net. "Immunocore and the Bill & Melinda Gates Foundation Collaborate to Develop Immunotherapies for Infectious Diseases". markets.businessinsider.com. Retrieved 10 November 2017.
- "Immunocore Starts Clinical Trial with IMCgp100 in Combination with MedImmune Immunotherapies Durvalumab and Tremelimumab | News - Immunocore". www.immunocore.com. Retrieved 10 November 2017.
- "Immunocore and Lilly announce immunotherapy-based clinical trial collaboration in melanoma | News - Immunocore". www.immunocore.com. Retrieved 10 November 2017.
- Slatko, Joshua (December 2013). "BMS changes senior management team". People on the Move: Biopharma. Med Ad News. p. 27.
- "Immunocore appoints Dr Eliot Forster as Chief Executive Officer | News - Immunocore". www.immunocore.com. Retrieved 10 November 2017.
- "Immunocore Announces Leadership Change | News - Immunocore". www.immunocore.com. Retrieved 27 February 2018.