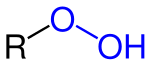
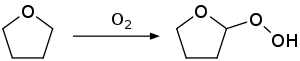Hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.[1]
Properties
The O−O bond length in peroxides is about 1.45 Å, and the R−O−O angles (R = H, C) are about 110° (water-like). Characteristically, the C−O−O−H dihedral angles are about 120°. The O−O bond is relatively weak, with a bond dissociation energy of 45–50 kcal/mol (190–210 kJ/mol), less than half the strengths of C−C, C−H, and C−O bonds.[2][3]
Use and reactions
Hydroperoxides can be reduced to alcohols with lithium aluminium hydride, as described in this idealized equation:
- 4 ROOH + LiAlH4 → LiAlO2 + 2 H2O + 4 ROH
This reaction is the basis of methods for analysis of organic peroxides.[4] Another way to evaluate the content of peracids and peroxides is the volumetric titration with alkoxides such as sodium ethoxide.[5] The phosphite esters and tertiary phosphines also effect reduction:
- ROOH + PR3 → OPR3 + ROH
Hydroperoxides are intermediates in the production of many organic compounds in industry. For example, the cobalt catalyzed oxidation of cyclohexane to cyclohexanone:[6]
- C6H12 + O2 → (CH2)5CO + H2O
Acetone and phenol are produced by the so-called cumene process, which proceeds via cumene hydroperoxide.
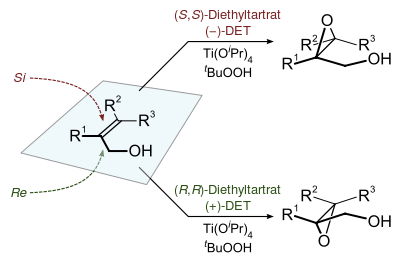
Many epoxides are prepared using hydroperoxides as reagents, such as the Halcon process for the production of propylene oxide. The Sharpless epoxidation is a related reaction conducted on laboratory scale. tert-Butyl hydroperoxide (TBHP) is an organic-soluble oxidant employed in these operations.[7]
Drying oils, as found in many paints and varnishes, function via the formation of hydroperoxides.
Formation
Hydroperoxides play important roles in biology. Hundreds of hydroperoxides are known, being derived from fatty acids, steroids, and terpenes. The biosynthesis of these species is effected extensively by enzymes.


Compounds with allylic and benzylic C−H bonds are especially susceptible to oxygenation.[9] Such reactivity is exploited industrially on a very large scale for the production of phenol by the Cumene process or Hock process for its cumene and cumene hydroperoxide intermediates.[10] Such reactions rely on radical initiators that reacts with oxygen to form an intermediate that abstracts a hydrogen atom from a weak C-H bond. The resulting radical binds O2, to give hydroperoxyl (ROO.), which then continues the cycle of H-atom abstraction.[11]
Auto-oxidation reaction is observed with common ethers, such as diethyl ether, diisopropyl ether, tetrahydrofuran or 1,4-dioxane. An illustrative product is diethyl ether peroxide. Such compounds can result in a serious explosion. The common solvent tetrahydrofuran illustrates this tendency to form highly explosive hydroperoxide on storage in air.[11]
To minimize this problem, commercial samples of THF are often inhibited with butylated hydroxytoluene (BHT). Distillation of THF to dryness is avoided because the explosive peroxides concentrate in the residue.

References
- Klenk, Herbert; Götz, Peter H.; Siegmeier, Rainer; Mayr, Wilfried. "Peroxy Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
- Bach, Robert D.; Ayala, Philippe Y.; Schlegel, H. B. (1996). "A Reassessment of the Bond Dissociation Energies of Peroxides. An ab Initio Study". J. Am. Chem. Soc. 118 (50): 12758–12765. doi:10.1021/ja961838i.
- Otto Exner (1983). "Stereochemical and conformational aspects of peroxy compounds". In Saul Patai (ed.). PATAI'S Chemistry of Functional Groups. Wiley. pp. 85–96. doi:10.1002/9780470771730.ch2. ISBN 9780470771730.
- Higuchi, T.; Zuck, Donald Anton (1951). "Behaviors of Several Compounds as Indicators in Lithium Aluminum Hydride Titration of Functional Groups". Journal of the American Chemical Society. 73 (6): 2676. doi:10.1021/ja01150a073.
- Martin, A. J. (1957). "Potentiometric titration of hydroperoxide and peracid in Anhydrous Ethylenediamine". Analytical Chemistry. 29: 79–81. doi:10.1021/ac60121a022.
- Michael T. Musser (2005). "Cyclohexanol and Cyclohexanone". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_217.CS1 maint: uses authors parameter (link)
- Hill, J. G.; Sharpless, K. B.; Exon, C. M.; Regenye, R. (1985). "Enantioselective Epoxidation Of Allylic Alcohols: (2s,3s)-3-propyloxiranemethanol". Org. Synth. 63: 66. doi:10.15227/orgsyn.063.0066.CS1 maint: uses authors parameter (link)
- Matsui K (2006). "Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism". Current Opinion in Plant Biology. 9 (3): 274–80. doi:10.1016/j.pbi.2006.03.002. PMID 16595187.
- Knight, H. B.; Swern, Daniel (1954). "Tetralin Hydroperoxide". Org. Synth. 34: 90. doi:10.15227/orgsyn.034.0090..
- Brückner, R. Reaktionsmechanismen: organische Reaktionen, Stereochemie, moderne Synthesemethoden, pp. 41–42, Spektrum Akademischer Verlag, Munich, 2004, ISBN 3-8274-1579-9 (in German)
- Heinz G. O. Becker Organikum, Wiley-VCH, 2001, ISBN 3-527-29985-8 pp. 206–207