Humus
In soil science, humus (derived in 1790–1800 from the Latin humus for earth, ground[1]) denominates the fraction of soil organic matter that is amorphous and without the "cellular cake structure characteristic of plants, micro-organisms or animals".[2] Humus significantly affects the bulk density of soil and contributes to its retention of moisture and nutrients. Even though compost and humus are used interchangeably, they are not the same. The difference with compost is that humus is created through anaerobic fermentation, while the first is made posible by aerobic decomposition.
In agriculture, "humus" sometimes also is used to describe mature or natural compost extracted from a woodland or other spontaneous source for use as a soil conditioner.[3] It is also used to describe a topsoil horizon that contains organic matter (humus type,[4] humus form,[5] humus profile).[6]
Humus is the dark organic matter that forms in soil when dead plant and animal matter decays. Humus has many nutrients that improve the health of soil, nitrogen being the most important. The ratio of carbon to nitrogen (C:N) of humus is 10:1.
Description
It is difficult to define humus precisely because it is a very complex substance which is not fully understood. Humus is different from decomposing soil organic matter. The latter looks rough and has visible remains of the original plant or animal matter. Fully humified humus, on the contrary, has a uniformly dark, spongy, and jelly-like appearance, and is amorphous; it may gradually decompose over several years or persist for millennia.[7] It has no determinate shape, structure, or quality. However, when examined under a microscope, humus may reveal tiny plant, animal, or microbial remains that have been mechanically, but not chemically, degraded.[8] This suggests an ambiguous boundary between humus and soil organic matter. While distinct, humus is an integral part of soil organic matter.[9]
Humification
Microorganisms decompose a large portion of the soil organic matter into inorganic minerals that the roots of plants can absorb as nutrients. This process is termed "mineralization". In this process, nitrogen (nitrogen cycle) and the other nutrients (nutrient cycle) in the decomposed organic matter are recycled. Depending on the conditions in which the decomposition occurs, a fraction of the organic matter does not mineralize, and instead is transformed by a process called "humification" into concatenations of organic polymers. Because these organic polymers are resistant to the action of microorganisms, they are stable, and constitute humus. This stability implies that humus integrates into the permanent structure of the soil, thereby improving it.[10]
Humification can occur naturally in soil or artificially in the production of compost. Organic matter is humified by a combination of saprotrophic fungi, bacteria, microbes and animals such as earthworms, nematodes, protozoa, and arthropods.[11] Plant remains, including those that animals digested and excreted, contain organic compounds: sugars, starches, proteins, carbohydrates, lignins, waxes, resins, and organic acids. Decay in the soil begins with the decomposition of sugars and starches from carbohydrates, which decompose easily as detritivores initially invade the dead plant organs, while the remaining cellulose and lignin decompose more slowly.[12] Simple proteins, organic acids, starches, and sugars decompose rapidly, while crude proteins, fats, waxes, and resins remain relatively unchanged for longer periods of time. Lignin, which is quickly transformed by white-rot fungi,[13] is one of the primary precursors of humus,[14] together with by-products of microbial[15] and animal[16] activity. The humus produced by humification is thus a mixture of compounds and complex biological chemicals of plant, animal, or microbial origin that has many functions and benefits in soil. Some judge earthworm humus (vermicompost) to be the optimal organic manure.[17]
Stability
Much of the humus in most soils has persisted for more than 100 years, rather than having been decomposed into CO2, and can be regarded as stable; this organic matter has been protected from decomposition by microbial or enzyme action because it is hidden (occluded) inside small aggregates of soil particles, or tightly sorbed or complexed to clays.[18] Most humus that is not protected in this way is decomposed within 10 years and can be regarded as less stable or more labile. Stable humus contributes few plant-available nutrients in soil, but it helps maintain its physical structure.[19] A very stable form of humus is formed from the slow oxidation of soil carbon after the incorporation of finely powdered charcoal into the topsoil. This process is speculated to have been important in the formation of the very fertile Amazonian terra preta do Indio.[20]
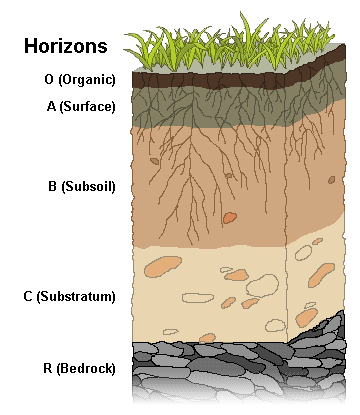
Horizons
Humus has a characteristic black or dark brown color and is organic due to an accumulation of organic carbon. Soil scientists use the capital letters O, A, B, C, and E to identify the master horizons, and lowercase letters for distinctions of these horizons. Most soils have three major horizons: the surface horizon (A), the subsoil (B), and the substratum (C). Some soils have an organic horizon (O) on the surface, but this horizon can also be buried. The master horizon (E) is used for subsurface horizons that have significantly lost minerals (eluviation). Bedrock, which is not soil, uses the letter R.
Benefits of soil organic matter and humus
The importance of chemically stable humus is thought by some to be the fertility it provides to soils in both a physical and chemical sense,[21] though some agricultural experts put a greater focus on other features of it, such as its ability to suppress disease.[22] It helps the soil retain moisture[23] by increasing microporosity,[24] and encourages the formation of good soil structure.[25][26] The incorporation of oxygen into large organic molecular assemblages generates many active, negatively charged sites that bind to positively charged ions (cations) of plant nutrients, making them more available to the plant by way of ion exchange.[27] Humus allows soil organisms to feed and reproduce, and is often described as the "life-force" of the soil.[28][29]
- The process that converts soil organic matter into humus feeds the population of microorganisms and other creatures in the soil, and thus maintains high and healthy levels of soil life.[28][29]
- The rate at which soil organic matter is converted into humus promotes (when fast) or limits (when slow) the coexistence of plants, animals, and microorganisms in the soil.
- Effective humus and stable humus are additional sources of nutrients for microbes: the former provides a readily available supply and the latter acts as a longeval storage reservoir.
- Decomposition of dead plant material causes complex organic compounds to be slowly oxidized (lignin-like humus) or to decompose into simpler forms (sugars and amino sugars, and aliphatic and phenolic organic acids), which are further transformed into microbial biomass (microbial humus) or reorganized, and further oxidized, into humic assemblages (fulvic acids and humic acids), which bind to clay minerals and metal hydroxides. The ability of plants to absorb humic substances with their roots and metabolize them has been long debated. There is now a consensus that humus functions hormonally rather than simply nutritionally in plant physiology.[30][31]
- Humus is a colloidal substance and increases the cation exchange capacity of soil, hence its ability to store nutrients by chelation. While these nutrient cations are available to plants, they are held in the soil and prevented from being leached by rain or irrigation.[27]
- Humus can hold the equivalent of 80–90% of its weight in moisture, and therefore increases the soil's capacity to withstand drought.[32][33]
- The biochemical structure of humus enables it to moderate, i.e. buffer, excessive acidic or alkaline soil conditions.[34]
- During humification, microbes secrete sticky, gum-like mucilages; these contribute to the crumby structure (tilth) of the soil by adhering particles together and allowing greater aeration of the soil.[35] Toxic substances such as heavy metals and excess nutrients can be chelated, i. e., bound to the organic molecules of humus, and so prevented from leaching away.[36]
- The dark, usually brown or black, color of humus helps to warm cold soils in Spring.
See also
References
- "Humus." Dictionary.com Unabridged, version 1.1. Random House, Inc., 23 Sep 2008, in .
- Whitehead, D. C.; Tinsley, J. (1963). "The biochemistry of humus formation". Journal of the Science of Food and Agriculture. 14 (12): 849–857. doi:10.1002/jsfa.2740141201.
- "Humus." Encyclopaedia Britannica. Encyclopaedia Britannica Online. Encyclopaedia Britannica Inc., 2011. Accessed 24 Nov 2011. <http://www.britannica.com/EBchecked/topic/276408/humus>.
- Chertov, O.G.; Kornarov, A.S.; Crocker, G.; Grace, P.; Klir, J.; Körschens, M.; Poulton, P.R.; Richter, D. (1997). "Simulating trends of soil organic carbon in seven long-term experiments using the SOMM model of the humus types". Geoderma. 81 (1–2): 121–135. Bibcode:1997Geode..81..121C. doi:10.1016/S0016-7061(97)00085-2.
- Baritz, R., 2003. Humus forms in forests of the northern German lowlands. Schweizerbart, Stuttgart, Germany, 145 pp
- Bunting, B.T.; Lundberg, J. (1995). "The humus profile-concept, class and reality". Geoderma. 40 (1–2): 17–36. Bibcode:1987Geode..40...17B. doi:10.1016/0016-7061(87)90011-5.
- Di Giovanni1, C.; Disnar, J.R.; Bichet, V.; Campy, M. (1998). "Sur la présence de matières organiques mésocénozoïques dans des humus actuels (bassin de Chaillexon, Doubs, France)". Comptes Rendus de l'Académie des Sciences, Série IIA. 326 (8): 553–559. Bibcode:1998CRASE.326..553D. doi:10.1016/S1251-8050(98)80206-1.
- Nicolas Bernier and Jean-François Ponge (1994). "Humus form dynamics during the sylvogenetic cycle in a mountain spruce forest" (PDF). Soil Biology and Biochemistry. 26 (2): 183–220. CiteSeerX 10.1.1.635.6402. doi:10.1016/0038-0717(94)90161-9.
- "Humintech® | Definition of Soil Organic Matter & Humic Acids Based Products". Archived from the original on 21 September 2015. Retrieved 5 April 2009.
- Brady, N. C.; Weil, R. R. (1999). The nature and properties of soils. Upper Saddle River, N. J.: Prentice Hall, Inc.
- Soil biology
- Berg, B., McClaugherty, C., 2007. Plant litter: decomposition, humus formation, carbon sequestration, 2nd ed. Springer, 338 pp., ISBN 3-540-74922-5
- Levin, L.; Forchiassin, F.; Ramos, A.M. (2002). "Copper induction of lignin-modifying enzymes in the white-rot fungus Trametes trogii". Mycologia. 94 (3): 377–383. doi:10.2307/3761771. JSTOR 3761771.
- González-Pérez, M.; Vidal Torrado, P.; Colnago, L.A.; Martin-Neto, L.; Otero, X.L.; Milori, D.M.B.P.; Haenel Gomes, F. (2008). "13C NMR and FTIR spectroscopy characterization of humic acids in spodosols under tropical rain forest in southeastern Brazil". Geoderma. 146 (3–4): 425–433. Bibcode:2008Geode.146..425G. doi:10.1016/j.geoderma.2008.06.018.
- Knicker, H.; Almendros, G.; González-Vila, F.J.; Lüdemann, H.D.; Martin, F. (1995). "13C and 15N NMR analysis of some fungal melanins in comparison with soil organic matter". Organic Geochemistry. 23 (11–12): 1023–1028. doi:10.1016/0146-6380(95)00094-1.
- Muscoloa, A.; Bovalob, F.; Gionfriddob, F.; Nardi, S. (1999). "Earthworm humic matter produces auxin-like effects on Daucus carota cell growth and nitrate metabolism". Soil Biology and Biochemistry. 31 (9): 1303–1311. doi:10.1016/S0038-0717(99)00049-8.
- "Vermiculture". Archived from the original on 17 January 2016. Retrieved 17 April 2009.
- Dungait, J. A.; Hopkins, D. W.; Gregory, A. S.; Whitmore, A. P. (2012). "Soil organic matter turnover is governed by accessibility not recalcitrance" (PDF). Global Change Biology. 18 (6): 1781–1796. Bibcode:2012GCBio..18.1781D. doi:10.1111/j.1365-2486.2012.02665.x. Retrieved 30 August 2014.
- Oades, J. M. (1984). "Soil organic matter and structural stability: mechanisms and implications for management". Plant and Soil. 76 (1–3): 319–337. doi:10.1007/BF02205590.
- Lehmann, J., Kern, D.C., Glaser, B., Woods, W.I., 2004. Amazonian Dark Earths: origin, properties, management. Springer, 523 pp. ISBN 978-1-4020-1839-8
- Hargitai, L (1993). "The soil of organic matter content and humus quality in the maintenance of soil fertility and in environmental protection". Landscape and Urban Planning. 27 (2–4): 161–167. doi:10.1016/0169-2046(93)90044-E.
- Hoitink, H.A.; Fahy, P.C. (1986). "Basic for the control of soilborne plant pathogens with composts". Annual Review of Phytopathology. 24: 93–114. doi:10.1146/annurev.py.24.090186.000521.
- C.Michael Hogan. 2010. Abiotic factor. Encyclopedia of Earth. eds Emily Monosson and C. Cleveland. National Council for Science and the Environment Archived 8 June 2013 at the Wayback Machine. Washington DC
- De Macedo, J.R.; Do Amaral, Meneguelli; Ottoni, T.B.; Araujo, Jorge Araújo; de Sousa Lima, J. (2002). "Estimation of field capacity and moisture retention based on regression analysis involving chemical and physical properties in Alfisols and Ultisols of the state of Rio de Janeiro". Communications in Soil Science and Plant Analysis. 33 (13–14): 2037–2055. doi:10.1081/CSS-120005747.
- Hempfling, R.; Schulten, H.R.; Horn, R. (1990). "Relevance of humus composition to the physical/mechanical stability of agricultural soils: a study by direct pyrolysis-mass spectrometry". Journal of Analytical and Applied Pyrolysis. 17 (3): 275–281. doi:10.1016/0165-2370(90)85016-G.
- Soil Development: Soil Properties Archived 28 November 2012 at the Wayback Machine
- Szalay, A (1964). "Cation exchange properties of humic acids and their importance in the geochemical enrichment of UO2++ and other cations". Geochimica et Cosmochimica Acta. 28 (10): 1605–1614. Bibcode:1964GeCoA..28.1605S. doi:10.1016/0016-7037(64)90009-2.
- Elo, S.; Maunuksela, L.; Salkinoja-Salonen, M.; Smolander, A.; Haahtela, K. (2006). "Humus bacteria of Norway spruce stands: plant growth promoting properties and birch, red fescue and alder colonizing capacity". FEMS Microbiology Ecology. 31 (2): 143–152. doi:10.1111/j.1574-6941.2000.tb00679.x. PMID 10640667.
- Vreeken-Buijs, M.J.; Hassink, J.; Brussaard, L. (1998). "Relationships of soil microarthropod biomass with organic matter and pore size distribution in soils under different land use". Soil Biology and Biochemistry. 30: 97–106. doi:10.1016/S0038-0717(97)00064-3.
- Eyheraguibel, B.; Silvestrea, J. Morard (2008). "Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize" (PDF). Bioresource Technology. 99 (10): 4206–4212. doi:10.1016/j.biortech.2007.08.082. PMID 17962015.
- Zandonadi, D. B.; Santos, M. P.; Busato, J. G.; Peres, L. E. P.; Façanha, A. R. (2013). "Plant physiology as affected by humified organic matter". Theoretical and Experimental Plant Physiology. 25: 13–25. doi:10.1590/S2197-00252013000100003.
- Olness, A.; Archer, D. (2005). "Effect of organic carbon on available water in soil". Soil Science. 170 (2): 90–101. doi:10.1097/00010694-200502000-00002.
- Effect of Organic Carbon on Available Water in Soil : Soil Science
- Kikuchi, R (2004). "Deacidification effect of the litter layer on forest soil during snowmelt runoff: laboratory experiment and its basic formularization for simulation modeling". Chemosphere. 54 (8): 1163–1169. Bibcode:2004Chmsp..54.1163K. doi:10.1016/j.chemosphere.2003.10.025. PMID 14664845.
- Caesar-Tonthat, T.C. (2002). "Soil binding properties of mucilage produced by a basidiomycete fungus in a model system". Mycological Research (Submitted manuscript). 106 (8): 930–937. doi:10.1017/S0953756202006330.
- Huang, D.L.; Zeng, G.M.; Feng, C.L.; Hu, S.; Jiang, X.Y.; Tang, L.; Su, F.F.; Zhang, Y.; Zeng, W.; Liu, H.L. (2008). "Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity". Environmental Science and Technology. 42 (13): 4946–4951. Bibcode:2008EnST...42.4946H. doi:10.1021/es800072c. PMID 18678031.
External links
- Jerzy Weber. "Types of humus in soils". Agricultural University of Wroclaw, Poland. Retrieved 12 December 2013.
- Wershaw, R.L. "Evaluation of conceptual models of natural organic matter (humus) from a consideration of the chemical and biochemical processes of humification" (PDF). pubs.usgs.gov. USGS. Retrieved 14 March 2016.
- IHSS. "What are Humic Substances?". International Humic Substances Society. Retrieved 19 February 2018.