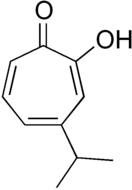
Hinokitiol
Hinokitiol (β-thujaplicin) is a natural monoterpenoid found in the wood of trees in the family Cupressaceae. It is a tropolone derivative and one of the thujaplicins.[2] Hinokitiol is widely used in oral care and treatment products for its broad spectrum anti-viral,[3] antimicrobial[4] and anti-inflammatory[5] action. Hinokitiol is a Zinc and Iron Ionophore, additionally it is approved as a food additive.[6]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Hydroxy-6-propan-2-ylcyclohepta-2,4,6-trien-1-one | |||
| Other names
β-Thujaplicin; 4-Isopropyltropolone | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.165 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C10H12O2 | |||
| Molar mass | 164.204 g·mol−1 | ||
| Appearance | Colorless to pale yellow crystals | ||
| Melting point | 50 to 52 °C (122 to 126 °F; 323 to 325 K) | ||
| Boiling point | 140 °C (284 °F; 413 K) at 10 mmHg | ||
| Hazards | |||
| Flash point | 140 °C (284 °F; 413 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The name of Hinokitiol originates from the fact it was originally isolated in Taiwanese hinoki in 1936.[7] It is actually almost absent in Japanese hinoki while it is contained in high concentration (about 0.04% of heartwood mass) in Juniperus cedrus, Hiba cedar wood (Thujopsis dolabrata) and Western red cedar (Thuja plicata). It can be readily extracted from the cedarwood with solvent and ultrasonication.[8]
Hinokitiol is structurally related to tropolone, which lacks the isopropyl substituent. Tropolones are well-known chelating agents.
Antimicrobial activity
Hinokitiol has a broad range of biological activities, many of which have been explored and characterised in literature. The first, and most well known, is the potent antimicrobial activity against many bacteria and fungi, regardless of antibiotic resistance.[9][10] Specifically, Hinokitiol has been shown to be effective against Streptococcus pneumoniae, Streptococcus mutans and Staphylococcus Aureus, common human pathogens.[11][12] Additionally, Hinokitiol has been shown to possess inhibitory effects on Chlamydia trachomatis and may be clinically useful as a topical drug.[13][14]
Antiviral activity
More recent studies have shown that Hinokitiol also demonstrates anti-viral action when used in combination with a zinc compound against a several human viruses including rhinovirus, coxsackievirus and mengovirus.[15] Curing viral infections has the potential to reap mass economic benefit and must be of dire importance to global institutions such as the World Health Organisation. By impairing viral polyprotein processing, Hinokitiol inhibits viral replication - however, this capability is dependent upon the availability of divalent metal ions, as Hinokitiol is a chelator thereof.[16] The presence of zinc in combination with Hinokitiol supports these capabilities and is discussed below.
Other activities
In addition to broad-spectrum antimicrobial activity, Hinokitiol also possesses anti-inflammatory and anti-tumour activities, characterised in a number of in vitro cell studies and in vivo animal studies. Hinokitiol inhibits key inflammatory markers and pathways, such as TNF-a and NF-kB, and its potential for treatment of chronic inflammatory or autoimmune conditions is being explored. Hinokitiol was found to exert cytotoxicity on several prominent cancer cell lines by inducing autophagic processes.[17][18]
Coronavirus research
Potential anti-viral effects of Hinokitiol arise from its action as a zinc ionophore. Hinokitiol enables the influx of zinc ions into cells, which inhibit the replication machinery of RNA viruses, and subsequently inhibiting the replication of the virus.[15] Some notable RNA viruses include the human influenza virus, SARS.[19] Zinc ions were able to significantly inhibit viral replication within cells, and proved that the action was dependent on zinc influx. This study was done with the zinc ionophore pyrithione, which functions very similarly to Hinokitiol.[19]
In cell cultures, hinokitiol inhibit human rhinovirus, coxsackievirus, and mengovirus multiplication. Hinokitiol interferes with the processing of viral polyproteins, thus inhibiting picornavirus replication. Hinokitiol inhibits the replication of picornaviruses by impairing the viral polyprotein processing and that the antiviral activity of hinokitiol is dependent on the availability of zinc ions.[20]
Iron ionophore
Hinokitiol has been shown to restore hemoglobin production in rodents. Hinokitiol acts as an iron ionophore to channel iron into cells,[21][22] increasing intracellular iron levels. Approximately 70% of the iron in humans is contained within red blood cells and specifically the hemoglobin protein. Iron is essential to almost all living organisms, and it is critical element of several anatomical functions such as the oxygen transport system, deoxyribonucleic acid (DNA) synthesis, and electron transport and an iron deficiency can lead to blood disorders such as Anemia which, can be significantly detrimental to both physical and mental performance.[23]
Zinc synergism
Hinokitiol is a Zinc ionophore and this capability is believed to inhibit viral replication. In brief, as a zinc ionophore, Hinokitiol aids in the transport of molecules into cells through a plasma membrane or intracellular membrane, thus increasing intracellular concentration of the specified molecule (ex. Zinc). Hence, by taking advantage of the antiviral properties of Zinc, in combination with Hinokitiol, Zinc uptake can be accelerated.[24]
Cancer research
In cell cultures and animal studies, hinokitiol has been shown to inhibit metathesis[25][26] and have anti-proliferative effects on cancer cells.[27][28][29][30][31][32]
Zinc Deficiency
Zinc deficiency has been demonstrated in some cancer cells and returning optimum intra-cellular zinc levels can lead to suppression tumor growth. Hinokitiol is a documented Zinc Ionophore, however more research is required at this time to establish effective concentrations of delivery methods for Hinokitiol and Zinc.
- "Effects of dietary zinc on melanoma growth and experimental metastasis..." [33]
- "Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature..." [34]
- "Association between serum zinc levels and lung cancer: a meta-analysis of observational studies..." [35]
- "Research progress on the relationship between zinc deficiency, related microRNA s, and esophageal carcinoma..." [36]
Products containing Hinokitiol

Hinokitiol is widely used in a range of consumer products including: cosmetics, toothpastes, oral sprays, sunscreens & hair-growth. One of the leading brands in the sale of consumer hinokitiol products is Hinoki Clinical. Hinoki Clinical (est. 1956) was established shortly after the first ‘industrial extraction of hinokitiol’ began in 1955.[37] Hinoki, currently has over 18 different product ranges with hinokitiol as an ingredient. Another brand, namely, 'Relief Life',[38] has boasted over a million sales with their ‘Dental Series’ toothpaste containing hinokitiol.[39] Other notable producers of hinokitiol based products include Otsuka Pharmaceuticals, Kobayashi Pharmaceuticals, Taisho Pharmaceuticals, SS Pharmaceuticals. Besides Asia, companies such as Swanson Vitamins® are commencing the utilisation of hinokitiol in consumer products in markets such as the U.S.A.[40] and Australia[41] as an anti-oxidant serum and in other endeavours. In 2006, hinokitiol was categorised under the Domestic Substances List in Canada as non-persistent, non-bioaccumulative & non-toxic to aquatic organisms.[42] The Environmental Working Group (EWG), an American activist group, has dedicated a page to the ingredient hinokitiol indicating that it is 'low hazard' in areas such as "Allergies & Immunotoxicity", "Cancer" & "Developmental & Reproductive Toxicity"[43] giving Hinokitiol a score of 1-2. In contrast to Hinokitiol's score, Propylparaben, an ingredient that is still sold in various mouthwashes, exhibits vast toxicity and hazardous concerns. Propylparaben has been deemed by the European Commission on Hormone Disruption to be a Human endocrine disruptor among other concerns[44] leaving it at a rating of 4-6 on the EWG website.
Dr ZinX
On 2 April 2020, Advance Nanotek,[45] an Australian producer of zinc oxide, filed a joint patent application with AstiVita Limited,[46] for an anti-viral composition that included various oral care products[47] containing hinokitiol as a vital component. The brand that is now incorporating this new invention is called Dr ZinX and is likely to release its Zinc + Hinokitiol combination in 2020.[48][49] On the 18th of May 2020 Dr ZinX published test results for a “Quantitative suspension test for evaluation of virucidal activity in the medical area”[50][51] returning a '3.25 log' reduction (99.9% reduction) for a neat concentration in 5 minutes against COVID-19 surrogate Feline Coronavirus.[52] Zinc is an essential dietary supplement and trace element in the body. Globally its is estimated that 17.3% of the population has inadequate Zinc intake.[53][54]
History
Discovery
Hinokitiol was discovered in 1936 by Dr. Tetsuo Nozoe from the essential oil component of Taiwan cypress. The discovery of this compound with a heptagonal molecular structure, which was said not to exist in nature, was globally recognised as a great achievement in the history of chemistry.[55]
Nozoe Tetsuo
Nozoe Tetsuo was born in Sendai, Japan on the 16th of May 1902. At the age of 21, he enrolled into a chemistry course at the Tohoku Imperial University in the department of chemistry.[56] After his graduation in March 1926, Nozoe stayed on as a research assistant but soon left Sendai for Formosa (Currently known as Taiwan) at the end of June 1926.[57]
Nozoe's main research interests lay in the study of natural products, especially those found in Formosa. Nozoe's documented work in Formosa concerned the chemical components of Taiwanhinoki, a native conifer growing in mountainous areas.[58] Nozoe determined a new compound, hinokitiol, from the components of this species and reported it for the first time in 1936 in a special issue of Bulletin of the Chemical Society of Japan.[59]
When a symposium, "Tropolone and Allied Compounds" was organised by the Chemical Society of London in November 1950, Nozoe's work on hinokitiol was mentioned as a pioneering contribution to tropolone chemistry, thereby helping Nozoe's research gain recognition in the West.[60] Nozoe was able to publish his work on hinokitiol and its derivatives in Nature in 1951 thanks to J.W. Cook, the chairman of the symposium. Nozoe's work, which began with research on natural products in Taiwan and became developed fully in Japan in the 1950s and the 60s, introduced a new field of organic chemistry, i.e., the chemistry of non-benzenoid aromatic compounds.[61] His work was well received in Japan and thus, Nozoe received the Order of Culture, the highest honour for contributing researchers and artists in 1958, at the age of 56.[62]
A Promising Future
Beginning in the 2000s, researchers recognised that hinokitiol could be of value as a pharmaceutical, notably for inhibiting the bacterium Chlamydia trachomatis.
Chemist Martin Burke and colleagues at the University of Illinois at Urbana–Champaign and at other institutions discovered a significant medical use for hinokitiol. Burke’s goal was to overcome irregular iron transport in animals. Insufficiencies of several proteins can lead to iron deficiency in cells (anemia) or the opposite effect, Hemochromatosis.[63] Using gene-depleted yeast cultures as surrogates, the researchers screened a library of small biomolecules for signs of iron transport and therefore cell growth. Hinokitiol popped up as the one that restored cell functionality. Further work by the team established the mechanism by which hinokitiol restores or reduces cell iron.[64] They then switched their study to mammals and found that when rodents that had been engineered to lack “iron proteins” were fed hinokitiol, they regained iron uptake in the gut. In a similar study on zebrafish, the molecule restored Hemoglobin production.[65] A commentary on the work of Burke et al. nicknamed hinokitiol the “Iron Man molecule”. This is fitting/ironic because discoverer Nozoe’s first name can be translated into English as “iron man”.
Significant research has also been conducted into the oral applications of Hinokitiol given the increased demand for Hinokitiol based oral products. One such study, affiliated with 8 different institutions in Japan, titled: "Antibacterial Activity of Hinokitiol Against Both Antibiotic-Resistant and -Susceptible Pathogenic Bacteria That Predominate in the Oral Cavity and Upper Airways" came to the conclusion that "hinokitiol exhibits antibacterial activity against a broad spectrum of pathogenic bacteria and has low cytotoxicity towards human epithelial cells."[12]
Relevant Studies
- "Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture..."[19]
- "Antiviral Activity of the Zinc Ionophores Pyrithione and Hinokitiol against Picornavirus Infections..."[66]
- "Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis..."[67]
- "High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa..."[68]
- "Antiviral Medication..."[69]
- “Antiviral Agent and Throat Candy, Gargle, and Mouthwash Using the Same...” [70]
- “Antibacterial and antifungal Activity Method, Therapeutic Method of Infectious Diseases and Preserving Method of Cosmetics...” [71]
- “Protective effect of hinokitiol against periodontal bone loss in ligature-induced experimental periodontitis in mice...”[72]
- "A New Antidiabetic Zn(II)–Hinokitiol (β-Thujaplicin) Complex with Zn(O4) Coordination Mode..." [73]
- "[Zn(hkt)2] (Zinc & Hinokitiol) exerted the main effect on peripheral organs by ameliorating insulin resistance..."[74]
References
- β-Thujaplicin at Sigma-Aldrich
- Chedgy RJ, Lim YW, Breuil C (May 2009). "Effects of leaching on fungal growth and decay of western redcedar". Canadian Journal of Microbiology. 55 (5): 578–86. doi:10.1139/W08-161. PMID 19483786.
- Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJ, Seipelt J (January 2009). "Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections". Journal of Virology. 83 (1): 58–64. doi:10.1128/JVI.01543-08. PMC 2612303. PMID 18922875.
- Inamori Y, Shinohara S, Tsujibo H, Okabe T, Morita Y, Sakagami Y, et al. (September 1999). "Antimicrobial activity and metalloprotease inhibition of hinokitiol-related compounds, the constituents of Thujopsis dolabrata S. and Z. hondai MAK". Biological & Pharmaceutical Bulletin. 22 (9): 990–3. doi:10.1248/bpb.22.990. PMID 10513629.
- Ye J, Xu YF, Lou LX, Jin K, Miao Q, Ye X, Xi Y (July 2015). "Anti-inflammatory effects of hinokitiol on human corneal epithelial cells: an in vitro study". Eye. 29 (7): 964–71. doi:10.1038/eye.2015.62. PMC 4506343. PMID 25952949.
- "Stress Check System". Health evaluation and promotion. 43 (2): 299–303. 2016. doi:10.7143/jhep.43.299. ISSN 1347-0086.
- Murata I, Itô S, Asao T (December 2012). "Tetsuo Nozoe: chemistry and life". Chemical Record. 12 (6): 599–607. doi:10.1002/tcr.201200024. PMID 23242794.
- Chedgy RJ, Daniels CR, Kadla J, Breuil C (2007). "Screening fungi tolerant to Western red cedar (Thuja plicata Donn) extractives. Part 1. Mild extraction by ultrasonication and quantification of extractives by reverse-phase HPLC". Holzforschung. 61 (2): 190–194. doi:10.1515/HF.2007.033.
- Shih YH, Chang KW, Hsia SM, Yu CC, Fuh LJ, Chi TY, Shieh TM (June 2013). "In vitro antimicrobial and anticancer potential of hinokitiol against oral pathogens and oral cancer cell lines". Microbiological Research. 168 (5): 254–62. doi:10.1016/j.micres.2012.12.007. PMID 23312825.
- Morita Y, Sakagami Y, Okabe T, Ohe T, Inamori Y, Ishida N (September 2007). "The mechanism of the bactericidal activity of hinokitiol". Biocontrol Science. 12 (3): 101–10. doi:10.4265/bio.12.101. PMID 17927050.
- Wang TH, Hsia SM, Wu CH, Ko SY, Chen MY, Shih YH, et al. (2016-09-28). "Evaluation of the Antibacterial Potential of Liquid and Vapor Phase Phenolic Essential Oil Compounds against Oral Microorganisms". PloS One. 11 (9): e0163147. Bibcode:2016PLoSO..1163147W. doi:10.1371/journal.pone.0163147. PMC 5040402. PMID 27681039.
- Domon H, Hiyoshi T, Maekawa T, Yonezawa D, Tamura H, Kawabata S, et al. (June 2019). "Antibacterial activity of hinokitiol against both antibiotic-resistant and -susceptible pathogenic bacteria that predominate in the oral cavity and upper airways". Microbiology and Immunology. 63 (6): 213–222. doi:10.1111/1348-0421.12688. PMID 31106894.
- Yamano H, Yamazaki T, Sato K, Shiga S, Hagiwara T, Ouchi K, Kishimoto T (June 2005). "In vitro inhibitory effects of hinokitiol on proliferation of Chlamydia trachomatis". Antimicrobial Agents and Chemotherapy. 49 (6): 2519–21. doi:10.1128/AAC.49.6.2519-2521.2005. PMC 1140513. PMID 15917561.
- Chedgy R (2010). Secondary metabolites of Western red cedar (Thuja plicata): their biotechnological applications and role in conferring natural durability. LAP Lambert Academic Publishing. ISBN 978-3-8383-4661-8.
- Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJ, Seipelt J (January 2009). "Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections". Journal of Virology. 83 (1): 58–64. doi:10.1128/JVI.01543-08. PMC 2612303. PMID 18922875.
- Krenn, B. M.; Gaudernak, E.; Holzer, B.; Lanke, K.; Van Kuppeveld, F. J. M.; Seipelt, J. (January 2009). "Antiviral Activity of the Zinc Ionophores Pyrithione and Hinokitiol against Picornavirus Infections". Journal of Virology. 83 (1): 58–64. doi:10.1128/JVI.01543-08. ISSN 0022-538X. PMC 2612303. PMID 18922875.
- Lee TB, Jun JH (2019-06-30). "Can Hinokitiol Kill Cancer Cells? Alternative Therapeutic Anticancer Agent via Autophagy and Apoptosis". Korean Journal of Clinical Laboratory Science. 51 (2): 221–234. doi:10.15324/kjcls.2019.51.2.221.
- Jayakumar T, Liu CH, Wu GY, Lee TY, Manubolu M, Hsieh CY, et al. (March 2018). "Hinokitiol Inhibits Migration of A549 Lung Cancer Cells via Suppression of MMPs and Induction of Antioxidant Enzymes and Apoptosis". International Journal of Molecular Sciences. 19 (4): 939. doi:10.3390/ijms19040939. PMC 5979393. PMID 29565268.
- te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ (November 2010). "Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture". PLoS Pathogens. 6 (11): e1001176. doi:10.1371/journal.ppat.1001176. PMC 2973827. PMID 21079686.
- Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJ, Seipelt J (January 2009). "Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections". Journal of Virology. 83 (1): 58–64. doi:10.1128/jvi.01543-08. PMC 2612303. PMID 18922875.
- Grillo AS, SantaMaria AM, Kafina MD, Cioffi AG, Huston NC, Han M, et al. (May 2017). "Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals". Science. 356 (6338): 608–616. doi:10.1126/science.aah3862. PMC 5470741. PMID 28495746.
- Service RF (2017-05-11). "Iron Man molecule restores balance to cells". Science. AAAS. doi:10.1126/science.aal1178.
- Abbaspour N, Hurrell R, Kelishadi R (February 2014). "Review on iron and its importance for human health". Journal of Research in Medical Sciences. 19 (2): 164–74. PMC 3999603. PMID 24778671.
- "Ionophores - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2020-06-25.
- Jayakumar T, Liu CH, Wu GY, Lee TY, Manubolu M, Hsieh CY, et al. (March 2018). "Hinokitiol Inhibits Migration of A549 Lung Cancer Cells via Suppression of MMPs and Induction of Antioxidant Enzymes and Apoptosis". International Journal of Molecular Sciences. 19 (4). doi:10.3390/ijms19040939. PMC 5979393. PMID 29565268.
- "Hinokitiol reduces tumor metastasis by inhibiting heparanase via extracellular signal-regulated kinase and protein kinase B pathway". www.medsci.org. Retrieved 2020-06-17.
- Lee TB, Jun JH (2019-06-30). "Can Hinokitiol Kill Cancer Cells? Alternative Therapeutic Anticancer Agent via Autophagy and Apoptosis". The Korean Journal of Clinical Laboratory Science. 51 (2): 221–234. doi:10.15324/kjcls.2019.51.2.221.
- Tu DG, Yu Y, Lee CH, Kuo YL, Lu YC, Tu CW, Chang WW (April 2016). "Hinokitiol inhibits vasculogenic mimicry activity of breast cancer stem/progenitor cells through proteasome-mediated degradation of epidermal growth factor receptor". Oncology Letters. 11 (4): 2934–2940. doi:10.3892/ol.2016.4300. PMC 4812586. PMID 27073579.
- Zhang G, He J, Ye X, Zhu J, Hu X, Shen M, et al. (March 2019). "β-Thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ROS-mediated Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma". Cell Death & Disease. 10 (4): 255. doi:10.1038/s41419-019-1492-6. PMID 30874538.
- Huang CH, Jayakumar T, Chang CC, Fong TH, Lu SH, Thomas PA, et al. (September 2015). "Hinokitiol Exerts Anticancer Activity through Downregulation of MMPs 9/2 and Enhancement of Catalase and SOD Enzymes: In Vivo Augmentation of Lung Histoarchitecture". Molecules. 20 (10): 17720–34. doi:10.3390/molecules201017720. PMID 26404213.
- Lee, Tae-Bok; Seo, Eun-Ju; Lee, Ji-Yun; Jun, Jin Hyun (2018-12-01). "Synergistic Anticancer Effects of Curcumin and Hinokitiol on Gefitinib Resistant Non-Small Cell Lung Cancer Cells". Natural Product Communications. 13 (12): 1934578X1801301223. doi:10.1177/1934578X1801301223.
- Shih YH, Chang KW, Hsia SM, Yu CC, Fuh LJ, Chi TY, Shieh TM (June 2013). "In vitro antimicrobial and anticancer potential of hinokitiol against oral pathogens and oral cancer cell lines". Microbiological Research. 168 (5): 254–62. doi:10.1016/j.micres.2012.12.007. PMID 23312825.
- Murray, Michael J.; Erickson, Kent L.; Fisher, Gerald L. (1983-12-01). "Effects of dietary zinc on melanoma growth and experimental metastasis". Cancer Letters. 21 (2): 183–194. doi:10.1016/0304-3835(83)90206-9. ISSN 0304-3835.
- Taccioli C, Chen H, Jiang Y, Liu XP, Huang K, Smalley KJ, et al. (October 2012). "Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature". Oncogene. 31 (42): 4550–8. doi:10.1038/onc.2011.592. PMID 22179833.
- Wang Y, Sun Z, Li A, Zhang Y (May 2019). "Association between serum zinc levels and lung cancer: a meta-analysis of observational studies". World Journal of Surgical Oncology. 17 (1): 78. doi:10.1186/s12957-019-1617-5. PMC 6503426. PMID 31060563.
- Liu CM, Liang D, Jin J, Li DJ, Zhang YC, Gao ZY, He YT (November 2017). "Research progress on the relationship between zinc deficiency, related microRNAs, and esophageal carcinoma". Thoracic Cancer. 8 (6): 549–557. doi:10.1111/1759-7714.12493. PMC 5668500. PMID 28892299.
- "Hinoki Clinical History". Hinoki Clinical. Retrieved 19 May 2020.
- "Real Life Product Line". Anshin Tsuuhan. Retrieved 19 May 2020.
- "Dental Series Product Page". Rakuten. Retrieved 19 May 2020.
- "Antioxidant Serum". Swanson Vitamins US. Retrieved 19 May 2020.
- "Antioxidant Serum AU". Swanson Vitamins Australia. Retrieved 19 May 2020.
- Secretariat, Treasury Board of Canada; Secretariat, Treasury Board of Canada. "Detailed categorization results of the Domestic Substances List - Open Government Portal". open.canada.ca. Retrieved 2020-06-17.
- "EWG Skin Deep® | What is HINOKITIOL". EWG. Retrieved 2020-06-17.
- "EWG Skin Deep® | What is PROPYLPARABEN". EWG. Retrieved 2020-06-26.
- "Advance NanoTek | Zinc Oxide Powder". Advance NanoTek. Retrieved 2020-05-20.
- "Health And Beauty | AstiVita". Health And Beauty | AstiVita. Retrieved 2020-05-20.
- "IP Australia: AusPat". Australian Government - IP Australia. Retrieved 2020-05-20.
- "Patent Update AstiVita" (PDF). Australian Stock Exchange. 20 May 2020.
- "Zinc + Hinokitiol". Dr ZinX. Retrieved 2020-05-20.
- Barrett M (18 May 2020). "AstiVita - Testing Results for Dr Zinx Zinc + Hinokitiol Combination" (PDF). ASX (Australian Stock Exchange). Retrieved 20 May 2020.
- Barrett M (18 May 2020). "Dr ZinX Test Results". Dr Zinx Oral Spray. Retrieved 20 May 2020.
- Administration, Australian Government Department of Health Therapeutic Goods (2020-05-07). "Surrogate viruses for use in disinfectant efficacy tests to justify claims against COVID-19". Therapeutic Goods Administration (TGA). Retrieved 2020-05-20.
- Wessells KR, Brown KH (2012-11-29). "Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting". PloS One. 7 (11): e50568. Bibcode:2012PLoSO...750568W. doi:10.1371/journal.pone.0050568. PMC 3510072. PMID 23209782.
- Ervin RB, Kennedy-Stephenson J (November 2002). "Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey". The Journal of Nutrition. 132 (11): 3422–7. doi:10.1093/jn/132.11.3422. PMID 12421862.
- "Hinokitiol Discovery". Hinoki. Retrieved 20 May 2020.
- Baosaree J, Rakharn N, Kammee D, Pengpajon P, Sriaphai S, Sittijanda S, et al. (2018-02-01). "The Effect of Rice Husk Charcoal and Sintering Temperature on Porosity of Sintered Mixture of Clay and Zeolite". Indian Journal of Science and Technology. 11 (8): 1–12. doi:10.17485/ijst/2018/v11i8/104310. ISSN 0974-5645.
- Murata I, Ito S, Toyonobu, Asao (2004). "Tesuo Nozoe (1902–1996". European Journal of Organic Chemistry. European Chemical Societies Publishing: 899–928.
- Nozoe T (March 1936). "Über eie Farbstoffe im Holzteile des "Hinokl"-Baumes. I. Hinokitin Und Hinokitiol (Vorläufige Mitteilung)". Bulletin of the Chemical Society of Japan. 11 (3): 295–298. doi:10.1246/bcsj.11.295.
- Fujimori K, Kaneko A, Kitamori Y, Aoki M, Makita M, Masuda N, Hokari K (November 1998). "Hinokitiol (β-Thujaplicin) from the Essential Oil of Hinoki [Chamaecyparis obtusa (Sieb. et Zucc.) Endl.]". Journal of Essential Oil Research. 10 (6): 711–712. doi:10.1080/10412905.1998.9701018. ISSN 1041-2905.
- Nozoe T (June 1951). "Substitution products of tropolone and allied compounds". Nature. 167 (4261): 1055–7. Bibcode:1951Natur.167.1055N. doi:10.1038/1671055a0. PMID 14843174.
- Kaji M (17 January 2018). "Development of the Natural Products Chemistry by Tetsuo Nozoe in Taiwan". Igniting the Chemical Ring of Fire. World Scientific. pp. 357–368. doi:10.1142/9781786344557_0012. ISBN 978-1-78634-454-0.
- Lo TB (February 2015). "Professor Tetsuo Nozoe and Taiwan". Chemical Record. 15 (1): 373–82. doi:10.1002/tcr.201402099. PMID 25597491.
- "Hinokitiol". American Chemical Society. Retrieved 2020-05-20.
- Grillo AS, SantaMaria AM, Kafina MD, Cioffi AG, Huston NC, Han M, et al. (May 2017). "Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals". Science. 356 (6338): 608–616. Bibcode:2017Sci...356..608G. doi:10.1126/science.aah3862. PMC 5470741. PMID 28495746.
- Service RF (May 2017). "Iron Man molecule restores balance to cells". Science Magazine. AAAS. Retrieved 2020-05-20.
- Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJ, Seipelt J (January 2009). "Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections". Journal of Virology. 83 (1): 58–64. doi:10.1128/JVI.01543-08. PMID 18922875.
- Wang WK, Chen SY, Liu IJ, Chen YC, Chen HL, Yang CF, et al. (July 2004). "Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis". Emerging Infectious Diseases. 10 (7): 1213–9. doi:10.3201/eid1007.031113. PMC 3323313. PMID 15324540.
- Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. (February 2020). "High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa". International Journal of Oral Science. 12 (1): 8. doi:10.1038/s41368-020-0074-x. PMC 7039956. PMID 32094336.
- Dong LC, Pollock-Dove C, Wong PS (2009), "CHRONSET™: An OROS® Delivery System for Chronotherapy", Chronopharmaceutics, John Wiley & Sons, Inc., pp. 175–186, doi:10.1002/9780470498392.ch8, ISBN 978-0-470-49839-2
- JA 2019077617, "Antiviral agent and throat candy, gargle, and mouthwash using the same", issued 2017-10-20
- Arima Y, Hatanaka A, Tsukihara S, Fujimoto K, Fukuda K, Sakurai H (1997). "Acavenging activities of α-, β-, γ-thujaplicins against active oxygen species". Chemical & Pharmaceutical Bulletin. 45 (12): 1881–1886. doi:10.1248/cpb.45.1881.
- Hiyoshi T, Domon H, Maekawa T, Yonezawa D, Kunitomo E, Tabeta K, Terao Y (April 2020). "Protective effect of hinokitiol against periodontal bone loss in ligature-induced experimental periodontitis in mice". Archives of Oral Biology. 112: 104679. doi:10.1016/j.archoralbio.2020.104679. PMID 32062102.
- Yamane M, Adachi Y, Yoshikawa Y, Sakurai H (December 2005). "A New Anti-diabetic Zn(II)–Hinokitiol (β-Thujaplicin) Complex with Zn(O 4 ) Coordination Mode". Chemistry Letters. 34 (12): 1694–1695. doi:10.1246/cl.2005.1694. ISSN 0366-7022.
- Naito Y, Yoshikawa Y, Shintani M, Kamoshida S, Kajiwara N, Yasui H (2017). "y Mice". Biological & Pharmaceutical Bulletin. 40 (3): 318–326. doi:10.1248/bpb.b16-00797. PMID 28250273.