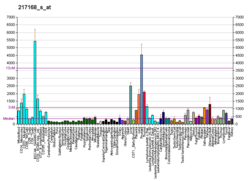
HERPUD1
Homocysteine-responsive endoplasmic reticulum-resident ubiquitin-like domain member 1 protein is a protein that in humans is encoded by the HERPUD1 gene.[5][6][7]
The accumulation of unfolded proteins in the endoplasmic reticulum (ER) triggers the ER stress response. This response includes the inhibition of translation to prevent further accumulation of unfolded proteins, the increased expression of proteins involved in polypeptide folding, known as the unfolded protein response (UPR), and the destruction of misfolded proteins by the ER-associated protein degradation (ERAD) system. This gene may play a role in both UPR and ERAD. Its expression is induced by UPR and it has an ER stress response element in its promoter region while the encoded protein has an N-terminal ubiquitin-like domain which may interact with the ERAD system. This protein has been shown to interact with presenilin proteins and to increase the level of amyloid-beta protein following its overexpression. Alternative splicing of this gene produces multiple transcript variants, some encoding different isoforms. The full-length nature of all transcript variants has not been determined.[7]
References
- GRCh38: Ensembl release 89: ENSG00000051108 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000031770 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Kokame K, Agarwala KL, Kato H, Miyata T (October 2000). "Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress". The Journal of Biological Chemistry. 275 (42): 32846–53. doi:10.1074/jbc.M002063200. PMID 10922362.
- van Laar T, Schouten T, Hoogervorst E, van Eck M, van der Eb AJ, Terleth C (March 2000). "The novel MMS-inducible gene Mif1/KIAA0025 is a target of the unfolded protein response pathway". FEBS Letters. 469 (1): 123–31. doi:10.1016/S0014-5793(00)01253-9. PMID 10708769.
- "Entrez Gene: HERPUD1 homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1".
- Kim TY, Kim E, Yoon SK, Yoon JB (May 2008). "Herp enhances ER-associated protein degradation by recruiting ubiquilins". Biochemical and Biophysical Research Communications. 369 (2): 741–6. doi:10.1016/j.bbrc.2008.02.086. PMID 18307982.
Further reading
- van Laar T, van der Eb AJ, Terleth C (June 2001). "Mif1: a missing link between the unfolded protein response pathway and ER-associated protein degradation?". Current Protein & Peptide Science. 2 (2): 169–90. doi:10.2174/1389203013381189. PMID 12370023.
- Nomura N, Miyajima N, Sazuka T, Tanaka A, Kawarabayasi Y, Sato S, et al. (1995). "Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001-KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid cell line KG-1". DNA Research. 1 (1): 27–35. doi:10.1093/dnares/1.1.27. PMID 7584026.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Andersson B, Wentland MA, Ricafrente JY, Liu W, Gibbs RA (April 1996). "A "double adaptor" method for improved shotgun library construction". Analytical Biochemistry. 236 (1): 107–13. doi:10.1006/abio.1996.0138. PMID 8619474.
- Yu W, Andersson B, Worley KC, Muzny DM, Ding Y, Liu W, et al. (April 1997). "Large-scale concatenation cDNA sequencing". Genome Research. 7 (4): 353–8. doi:10.1101/gr.7.4.353. PMC 139146. PMID 9110174.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Kokame K, Kato H, Miyata T (March 2001). "Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response". The Journal of Biological Chemistry. 276 (12): 9199–205. doi:10.1074/jbc.M010486200. PMID 11112790.
- Sai X, Kawamura Y, Kokame K, Yamaguchi H, Shiraishi H, Suzuki R, et al. (April 2002). "Endoplasmic reticulum stress-inducible protein, Herp, enhances presenilin-mediated generation of amyloid beta-protein". The Journal of Biological Chemistry. 277 (15): 12915–20. doi:10.1074/jbc.M112372200. PMID 11799129.
- Chtarbova S, Nimmrich I, Erdmann S, Herter P, Renner M, Kitajewski J, Müller O (November 2002). "Murine Nr4a1 and Herpud1 are up-regulated by Wnt-1, but the homologous human genes are independent from beta-catenin activation". The Biochemical Journal. 367 (Pt 3): 723–8. doi:10.1042/BJ20020699. PMC 1222938. PMID 12153396.
- Sai X, Kokame K, Shiraishi H, Kawamura Y, Miyata T, Yanagisawa K, Komano H (October 2003). "The ubiquitin-like domain of Herp is involved in Herp degradation, but not necessary for its enhancement of amyloid beta-protein generation". FEBS Letters. 553 (1–2): 151–6. doi:10.1016/S0014-5793(03)01009-3. PMID 14550564.
- Schulze A, Standera S, Buerger E, Kikkert M, van Voorden S, Wiertz E, et al. (December 2005). "The ubiquitin-domain protein HERP forms a complex with components of the endoplasmic reticulum associated degradation pathway". Journal of Molecular Biology. 354 (5): 1021–7. doi:10.1016/j.jmb.2005.10.020. PMID 16289116.
- Lim J, Hao T, Shaw C, Patel AJ, Szabó G, Rual JF, et al. (May 2006). "A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration". Cell. 125 (4): 801–14. doi:10.1016/j.cell.2006.03.032. PMID 16713569.
- Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn AC, et al. (November 2006). "Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element". Molecular and Cellular Biology. 26 (21): 7999–8010. doi:10.1128/MCB.01046-06. PMC 1636730. PMID 16940180.
- Lenz B, Bleich S, Beutler S, Schlierf B, Schwager K, Reulbach U, et al. (December 2006). "Homocysteine regulates expression of Herp by DNA methylation involving the AARE and CREB binding sites". Experimental Cell Research. 312 (20): 4049–55. doi:10.1016/j.yexcr.2006.09.004. PMID 17020760.