Girdler sulfide process
The Girdler sulfide (GS) process, also known as the Geib–Spevack (GS) process,[1] is an industrial production method for filtering out of natural water the heavy water (deuterium oxide = D2O) which is used in particle research, in Deuterium NMR spectroscopy, deuterated solvents for proton NMR spectroscopy, in heavy water nuclear reactors (as a coolant and moderator) and in deuterated drugs.
Karl-Hermann Geib and Jerome S. Spevack independently, and in parallel, invented the process in 1943[2] and its name derives from the Girdler company, which built the first American plant using the process.
The method is an isotopic exchange process between H2S and H2O ("light" water), that produces heavy water over several steps. It is a highly energy intensive process.[3] Seawater contains 180 parts per million of HDO.
Until its closure in 1997, the Bruce Heavy Water Plant in Ontario (located on the same site as Douglas Point and the Bruce Nuclear Generating Station) was the world's largest heavy water production plant, with a peak capacity of 1600 tonnes per year (800 tonnes per year per full plant, two fully operational plants at its peak). It used the Girdler sulfide process to produce heavy water, and required 340,000 tonnes (370,000 short tons) of feed water to produce 1 tonne (1.1 short tons) of heavy water.[4]
The first such facility of India's Heavy Water Board to use the Girdler process is at Rawatbhata near Kota, Rajasthan. This was followed by a larger plant at Manuguru, Andhra Pradesh. Other plants exist in the United States and Romania for example.[5]
The process
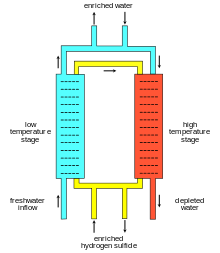
Each of a number of steps consists of two sieve tray columns. One column is maintained at 30 °C and is called the cold tower and the other at 130 °C and is called the hot tower. The enrichment process is based on the difference in separation between 30 °C and 130 °C.
The process of interest is the equilibrium reaction,
H2O + HDS ⇌ HDO + H2S
At 30 °C, the equilibrium constant K = 2.33, while at 130 °C, K = 1.82. This difference is exploited for enriching deuterium in heavy water.[6]
Hydrogen sulfide gas is circulated in a closed loop between the cold tower and the hot tower (although these can be separate towers, they can also be separate sections of one tower, with the cold section at the top). Demineralised and deaerated water is fed to the cold tower where deuterium migration preferentially takes place from the hydrogen sulfide gas to the liquid water. Normal water is fed to the hot tower where deuterium transfer takes place from the liquid water to the hydrogen sulfide gas. An appropriate "cascade" setup accomplishes enrichment: "enriched" water is fed into the cold tower and is further "enriched".
Normally in this process, water is enriched to 15–20% D2O. Further enrichment to "reactor-grade" heavy water (> 99% D2O) is done in another process, e.g. distillation.[7][8]
See also
References
- U.S. Patent 4,620,909, Method for isotope replenishment in an exchange liquid used in a laser induced isotope enrichment process
- Castell, Lutz (2003). Time, Quantum and Information. Google Books: Springer Science+Business Media. p. 37. ISBN 978-3-642-07892-7.
- Federation of American Scientists, Heavy Water Production, accessed February 1, 2007.
- "Bruce Heavy Water Plant Decommissioning" (PDF).
- "Heavy Water Board – A unit under Department of Atomic Energy, Govt. of India<". Archived from the original on October 12, 2007.
- Rae, H. K. (1978). "Selecting Heavy Water Processes". Separation of Hydrogen Isotopes. ACS Symposium Series. 68. pp. 1–26. doi:10.1021/bk-1978-0068.ch001. ISBN 978-0-8412-0420-1.
- Boris M. Andreev (2001). "Separating of Hydrogen Isotopes in H2O-H2S System". Separation Science and Technology. 36 (8–9): 1949–89. doi:10.1081/SS-100104764.
- "FAS Special Weapons Primer: Heavy water production".