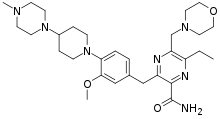
Gilteritinib
Gilteritinib (trade name Xospata) is an anti-cancer drug.[1] It acts as an inhibitor of AXL receptor tyrosine kinase, hence it is a tyrosine kinase inhibitor.[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Xospata |
| AHFS/Drugs.com | xospata |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C29H44N8O3 |
| Molar mass | 552.724 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It was developed by Astellas Pharma.
In April 2018, Astellas filed a new drug application with the Food and Drug Administration for gilteritinib for the treatment of adult patients with FLT3 mutation–positive relapsed or refractory acute myeloid leukemia (AML).[3]
In November 2018, the FDA approved gilteritinib for treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation as detected by an FDA-approved test.[4] The recommended dose of gilteritinib is 120 mg orally once daily.[5]
Gilteritinib was granted orphan drug status by the U.S. FDA, the European Commission (EC) and the Japan Ministry of Health, Labor and Welfare, for some AML patients.[6]
References
- Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. (August 2017). "Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study". The Lancet. Oncology. 18 (8): 1061–1075. doi:10.1016/S1470-2045(17)30416-3. PMC 5572576. PMID 28645776.
- Lee LY, Hernandez D, Rajkhowa T, Smith SC, Raman JR, Nguyen B, et al. (January 2017). "Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor". Blood. 129 (2): 257–260. doi:10.1182/blood-2016-10-745133. PMC 5234222. PMID 27908881.
- "FDA Approval Sought for Gilteritinib in FLT3+ AML". onclive.com. April 24, 2018. Retrieved September 29, 2018.
- U.S. Food and Drug Administration (2018-11-28). "FDA approves gilteritinib for relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation". www.fda.gov. Retrieved 2018-11-29.
- "Prescribing information for XOSPATA (gilteritinib)" (PDF). U.S. Food and Drug Administration.
- "U.S. FDA Grants Priority Review to Astellas' New Drug Application for Gilteritinib for the Treatment of Adult Patients with Relapsed or Refractory Acute Myeloid Leukemia (AML)". Drugs.com. Retrieved 2018-12-03.