Fluorosulfonate
Fluorosulfonate, in organic chemistry, is a functional group that has the chemical formula F-SO2-O-R, and typically is a very good leaving group. In inorganic chemistry, fluorosulfonate is another term for fluorosulfate, the anion F-SO2-O−, the conjugate base of fluorosulfonic acid. They form a series of salts with metal and organic cations called fluorosulfates.
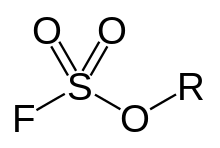
General chemical structure of a fluorosulfonate ester
Organic (alkyl) fluorosulfonates are usually strong alkylation agents, similar to triflate esters (F3C-SO2-OR). But unlike the triflate group, the fluorosulfonate group is not stable against hydrolysis. Therefore, fluorosulfonate esters are less frequently used as alkylation agents than triflate esters.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.