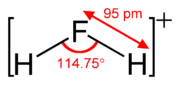
Fluoronium
The fluoronium ion is an inorganic cation with the chemical formula H
2F+
. The structure of the salt with the Sb
2F−
11 anion has been determined.[1] [2] The fluoronium ion is isoelectronic with the water molecule and the amide ion.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Fluoronium | |||
| Systematic IUPAC name
Fluoranium | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| |||
| |||
| Properties | |||
| H2F+ | |||
| Molar mass | 21.01428 g mol−1 | ||
| Conjugate base | Hydrogen fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
References
- Mootz, Dietrich; Bartmann, Klemens (1988). "The Fluoronium Ions H2F+ and H3F2+: Characterization by Crystal Structure Analysis". Angewandte Chemie. 27 (3): 391–392. doi:10.1002/anie.198803911.
- Diercksen, G. H. F.; von Niessen, W.; Kraemer, W. P. (1973). "SCF LCGO MO studies on the fluoronium ion FH+
2 and its hydrogen bonding interaction with hydrogen fluoride FH". Theoretical Chemistry Accounts: Theory, Computation, and Modeling (Theoretica Chimica Acta). 31 (3): 205–214. doi:10.1007/BF00526510.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.