Ferritic stainless steel
Ferritic stainless steel [1][2] forms one of the four stainless steel families, the other three being austenitic, martensitic and duplex stainless steels
History
Ferritic stainless steels were discovered early but it was only in the 1980s that the conditions were met for their growth:
- It was possible to obtain very low carbon levels at the steelmaking stage.
- Weldable grades were developed.
- Themomechanical processing solved the problems of "roping" and "ridging" that led to inhomogenous deformation during deep drawing and to textured surfaces.
- End-user markets (such as that of domestic appliances) demanded less expensive grades with a more stable price at a time when there were large variations of the price of nickel [3]. Ferritic stainless steel grades became attractive for some applications such as houseware.[4]
Metallurgy
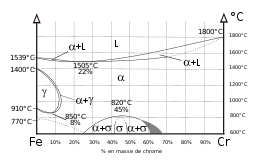
To qualify as stainless steel, Fe-base alloys must contain at least 10.5%Cr.
The Iron-Chromium phase diagram shows that up to about 13%Cr, the steel undergoes successive transfomations upon cooling from the liquid phase from ferritic α phase to austenitic γ phase and back to α. When some carbon is present, and if cooling occurs quickly, some of the austenite will transform into martensite.Tempering/annealing will transform the martensitic structure into ferrite and carbides.
Above about 17%Cr the steel will have a ferritic structure at all temperatures.
Above 25%Cr the Sigma phase may appear for relatively long times at temperature and induce room temperature embrittlement.
Chemical compositions of a few grades (main alloying elements)
| AISI / ASTM | EN | Weight % | |
|---|---|---|---|
| Cr | Other elements | ||
| 405 | 1.4000 | 12.0 - 14.0 | - |
| 409L | 1.4512 | 10.5 - 12.5 | 6(C+N)<Ti<0.65 |
| 410L | 1.4003 | 10.5 - 12.5 | 0.3<Ni<1.0 |
| 430 | 1.4016 | 16.0 - 18.0 | - |
| 439 | 1.4510 | 16.0 - 18.0 | 0.15+4(C+N)<Ti<0.8 |
| 430Ti | 1.4511 | 16.0 -18.0 | Ti: 0.6 |
| 441 | 1.4509 | 17.5 - 18.5 | 0.1<Ti<0.6
0.3+3C<Nb<1.0 |
| 434 | 1.4113 | 16.0 - 18.0 | 0.9<Mo<1.4 |
| 436 | 1.4513 | 16.0 - 18.0 | 0.9<Mo<1.4
0.3<Ti<0.6 |
| 444 | 1.4521 | 17.0 - 20.0 | 1.8<Mo<2.5
0.15+4(C+N)<Ti+Nb<0.8 |
| 447 | 1.4592 | 28 - 30.0 | 3.5<Mo<4.5
0.15+4(C+N)<Ti<0.8 |
Corrosion resistance
The pitting corrosion resistance of stainless steels is estimated by the pitting resistance equivalent number (PREN).
PREN = %Cr + 3.3%Mo + 16%N where the terms correspond to the contents by weight % of chromium, molybdenum and nitrogen respectively in the steel.
Nickel has no role in the pitting corrosion resistance, so ferritic stainless steels can be as resistant to this form of corrosion as austentitc grades.
In addition, ferritic grades are very resistant to stress corrosion cracking (SCC).
Physical properties
Ferritic stainless steels are magnetic
| AISI / ASTM | Density
g/cm3 |
Electrical
Resistance μΩ.m |
Thermal
Conductivity at 20 °C W/(m.°K) |
Specific Heat
0 - 100 °C J/(Kg.°K) |
Themal expansion
0 - 600 °C 10−6/°K |
Young's Modulus
GPa |
|---|---|---|---|---|---|---|
| 409 / 410 | 7.7 | 0.58 | 25 | 460 | 12 | 220 |
| 430 | 7.7 | 0.60 | 25 | 460 | 11.5 | 220 |
| 430Ti / 439 / 441 | 7.7 | 0.60 | 25 | 460 | 11.5 | 220 |
| 434 / 436 / 444 | 7.7 | 0.60 | 23 | 460 | 11.5 | 220 |
| 447 | 7.7 | 0.62 | 17 | 460 | 11 | 220 |
Compared to austenitic stainless steels, they offer a better thermal conductivity, a plus for applications such as heat exchangers
The thermal expansion coefficient, close to that of carbon steel, facilitates the welding to carbon steels
Mechanical properties
| ASTM A240 | EN 10088-2 | ||||||
|---|---|---|---|---|---|---|---|
| UTS
MPa, min |
0.2% Yield
Stress MPa, min |
Elongation
%, min |
UTS
MPa |
0.2% Yield
Stress MPa, min |
Elongation
%, min | ||
| 409 | 390 | 170 | 20 | 1.4512 | 380 - 560 | 220 | 25 |
| 410 | 415 | 205 | 20 | 1.4003 | 450 - 650 | 320 | 20 |
| 430 | 450 | 205 | 22 | 1.4016 | 450 - 600 | 280 | 18 |
| 439 | 415 | 205 | 22 | 1.4510 | 420 - 600 | 240 | 23 |
| 441 | 415 | 205 | 22 | 1.4509 | 430 - 630 | 250 | 18 |
| 434 | 450 | 240 | 22 | 1.4113 | 450 - 630 | 280 | 18 |
| 436 | 450 | 240 | 22 | 1.4526 | 480 -560 | 300 | 25 |
| 444 | 415 | 275 | 20 | 1.4521 | 420 - 640 | 320 | 20 |
Applications
- Lower-cost or recent-production kitchenware
- White goods
- Solar heaters
- Slate hooks
- Coins
References
- P Lacombe, B Baroux G Beranger editors (1990). Les Aciers Inoxydables. Les éditions de Physique. pp. Chaoters 14 and 15. ISBN 2-86883-142-7.
- "The ferritic Solution". 2007. ISBN 2-930069-51-1.
- Charles, J.; Mithieux, J.D.; Santacreu, P.; Peguet, L. (2009). "The ferritic family: The appropriété answer to nickel volatility?". Revue de métallurgie. 106: 124–139.
- Ronchi, Gaetano (2012). "Stainless Steel for House-ware". Metal Bulletin.