Fehling's solution
Fehling's solution is a chemical reagent used to differentiate between water-soluble carbohydrate and ketone functional groups, and as a test for reducing sugars and non-reducing sugars, supplementary to the Tollens' reagent test. The test was developed by German chemist Hermann von Fehling in 1849.[1]
Fehling's test, Left side negative, right side positive | |
| Classification | Colorimetric method |
|---|---|
| Analytes | Monosaccharides |
Laboratory preparation
Fehling's solution is prepared by combining two separate solutions: Fehling's A, which is a deep blue aqueous solution of copper(II) sulfate, and Fehling's B, which is a colorless solution of aqueous potassium sodium tartrate (also known as Rochelle salt) made strongly strong alkali with potassium hydroxide. These two solutions, stable separately, are combined when needed for the test because the copper(II) complex formed by their combination is not stable. The active reagent is bis(tartrate) complex of Cu2+, which serves as an oxidizing agent. The tartrate tetraanions serve as bidentate alkoxide ligands.
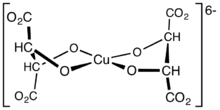 Structure of the main complex in Fehling's solution.
Structure of the main complex in Fehling's solution.
Use of the reagent
Fehling's solution can be used to distinguish aldehyde vs ketone functional groups. The compound to be tested is added to the Fehling's solution and the mixture is heated. Aldehydes are oxidized, giving a positive result, but ketones do not react, unless they are α-hydroxy ketones. The bistartratocuprate(II) complex oxidizes the aldehyde to a carboxylate anion, and in the process the copper(II) ions of the complex are reduced to copper(I) ions. Red copper(I) oxide then precipitates out of the reaction mixture, which indicates a positive result i.e. that redox has taken place (this is the same positive result as with Benedict's solution).
Fehling's test can be used as a generic test for monosaccharides and other reducing sugars (e.g., maltose). It will give a positive result for aldose monosaccharides (due to the oxidisable aldehyde group) but also for ketose monosaccharides, as they are converted to aldoses by the base in the reagent, and then give a positive result.[2]
Fehling's can be used to screen for glucose in urine, thus detecting diabetes. Another use is in the breakdown of starch to convert it to glucose syrup and maltodextrins in order to measure the amount of reducing sugar, thus revealing the dextrose equivalent (DE) of the starch sugar.
Formic acid (HCO2H) also gives a positive Fehling's test result, as it does with Tollens' test and Benedict's test also. The positive tests are consistent with it being readily oxidizable to carbon dioxide.
The solution cannot differentiate between benzaldehyde and acetone.
Net reaction
The net reaction between an aldehyde and the copper(II) ions in Fehling's solution may be written as:
- RCHO + 2 Cu2+ + 5 OH− → RCOO− + Cu2O + 3 H2O
or with the tartrate included:
- RCHO + 2 Cu(C4H4O6)22− + 5 OH− → RCOO− + Cu2O + 4 C4H4O62− + 3 H2O
See also
References
- H. Fehling (1849). "Die quantitative Bestimmung von Zucker und Stärkmehl mittelst Kupfervitriol" [The quantitative determination of sugar and starch by means of copper sulfate]. Annalen der Chemie und Pharmacie. 72 (1): 106–113. doi:10.1002/jlac.18490720112.
- Fehling's Test for Reducing Sugars
External links
