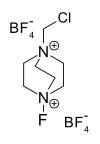Selectfluor
1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) or Selectfluor, a trademark of Air Products and Chemicals,[1] is a reagent in chemistry that is used as a fluorine donor. This compound is a derivative of the nucleophillic base DABCO. This colourless salt was first described in 1992[2] and has since been commercialized for use in organofluorine chemistry for electrophilic fluorination.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1-(Chloromethyl)-4-fluoro-1,4-diazabicyclo[2.2.2]octane-1,4-diium ditetrafluoroborate | |||
| Other names
F-TEDA, N-Chloromethyl-N-fluorotriethylenediammonium bis(tetrafluoroborate) | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.101.349 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H14B2ClF9N2 | |||
| Molar mass | 354.26 g/mol | ||
| Appearance | colourless solid | ||
| Melting point | 234 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
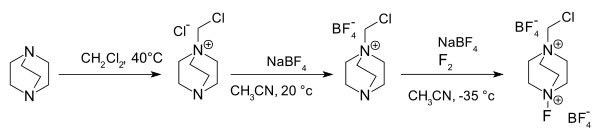
Preparation
Selectfluor is synthesized by the N-alkylation of diazabicyclo[2.2.2]octane (DABCO) with dichloromethane, followed by ion exchange with sodium tetrafluoroborate (replacing the chloride counterion for the tetrafluoroborate). Finally, this salt is treated with elemental fluorine and sodium tetrafluoroborate:[2]
Mechanism
Electrophilic fluorinating reagents can operate via electron transfer pathways or an SN2 attack at fluorine.[3] By using a charge-spin separated probe,[4] it was possible to show that the electrophilic fluorination of stilbenes with Selectfluor proceeds through an SET/fluorine atom transfer mechanism.[5]
In addition to these electrophilic fluorinations, it has also been shown that Selectfluor can transfer fluorine to alkyl radicals.[6][7]
Applications
The conventional source of "electrophilic fluorine", i.e. the equivalent to the superelectrophile F+, is gaseous fluorine, which requires specialised equipment for manipulation. Selectfluor reagent is a salt, the use of which requires only routine procedures. Like F2, the salt delivers the equivalent of F+. It is mainly used in the synthesis of organofluorine compounds:[8][9][10][11]
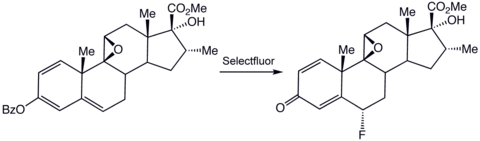
Specialized applications
Selectfluor reagent also serves as a strong oxidant, a property that is useful in other reactions in organic chemistry. Oxidation of alcohols and phenols. As applied to electrophilic iodination, Selectfluor reagent activates the I–I bond in I2 molecule.[12]
References
- Aitken, R. Alan; Carreira, Erick M. (2014). Science of Synthesis Knowledge Updates 2012, Volume 2. Stuttgart: Thieme Verlag. p. 494. ISBN 9783131788214.
- Banks, R. Eric; Mohialdin-Khaffaf, Suad N.; Lal, G. Sankar; Sharif, Iqbal; Syvret, Robert G. (1992). "1-Alkyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane salts: a novel family of electrophilic fluorinating agents". Journal of the Chemical Society, Chemical Communications (8): 595. doi:10.1039/C39920000595.
- Differring, Edmond; Wehrli, Markus (1991). "Nucleophilic substitution versus electron transfer: 2. SN2 at fluoride and electron transfer are competing and different pathways in electrophilic fluorinations". Tetrahedron Letters. 32 (31): 3819–3822. doi:10.1016/S0040-4039(00)79384-1. ISSN 0040-4039.
- Tojo, Sachiko; Morishima, Kazuhiro; Ishida, Akito; Majima, Tetsuro; Takamuku, Setsuo (1995). "Remarkable Enhancements of Isomerization and Oxidation of Radical Cations of Stilbene Derivatives Induced by Charge-Spin Separation". The Journal of Organic Chemistry. 60 (15): 4684–4685. doi:10.1021/jo00120a004. ISSN 0022-3263.
- Brandt, Jochen R.; Lee, Eunsung; Boursalian, Gregory B.; Ritter, Tobias (2014). "Mechanism of electrophilic fluorination with Pd(iv): fluoride capture and subsequent oxidative fluoride transfer". Chem. Sci. 5 (1): 169–179. doi:10.1039/C3SC52367E. ISSN 2041-6520. PMC 3870902. PMID 24376910.
- Rueda-Becerril, M.; Chatalova-Sazepin, C.; Leung, J. C. T.; Okbinoglu, T.; Kennepohl, P.; Paquin, J.-F.; Sammis, G. M. (2012). "Fluorine transfer to alkyl radicals". J. Am. Chem. Soc. 134 (9): 4026–4029. doi:10.1021/ja211679v. PMID 22320293.
- Paquin, J.-F.; Sammis, G.; Chatalova-Sazepin, C.; Hemelaere, R. (2015). "Recent advances in radical fluorination". Synthesis. 47 (17): 2554–2569. doi:10.1055/s-0034-1378824.
- Banks, R. Eric; Besheesh, Mohamed K.; Mohialdin-Khaffaf, Suad N.; Sharif, Iqbal (1996). "N-Halogeno compounds. Part 18. 1-Alkyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane salts: user-friendly site-selective electrophilic fluorinating agents of the N-fluoroammonium class". Journal of the Chemical Society, Perkin Transactions 1 (16): 2069–2076. doi:10.1039/P19960002069.
- Manral, Laxmi (2006). "Selectfluor (F-TEDA-BF4) C7H14B2ClF9N2". Synlett (5): 0807–0808. doi:10.1055/s-2006-933124.
- Stojan Stavbera and Marko Zupana (2005). "Selectfluortm F-TEDA-BF4 As a Versatile Mediator or Catalyst in Organic Chemistry" (PDF). Acta Chim. Slov. 52: 13–26.
- Singh, R.P.; Shreeve, J. M. (2004). "Recent Highlights in Electrophilic Fluorination with 1-Chloromethyl-4-Fluoro-1,4-Diazoniabicyclo[2.2.2]Octane Bis(Tetrafluoroborate)". Acc. Chem. Res. 37 (1): 31–44. doi:10.1021/ar030043v. PMID 14730992.
- Stavber, Stojan; Kralj, Petra; Zupan, Marko (2002-08-01). "Progressive Direct Iodination of Sterically Hindered Alkyl Substituted Benzenes". Synthesis. 2002 (11): 1513–1518. doi:10.1055/s-2002-33339. ISSN 0039-7881.
Other References
- Lal, G. S., J. Org. Chem. 1993, 58, 2791.
- Lal, G. S., Synth. Commun. 1995, 25 (5), 725.
- Banks, R. E.; Lawrence, N. J.; Popplewell, A. L., J. Chem. Soc., Chem. Commun. 1994, 343.
- Banks, R. E., J. Fluorine Chem. 1998, 87, 1.
- Zupan, M.; Iskra, J.; Stavber, S., J. Fluorine Chem., 1995, 70, 7.
- Matthews, D.P.; Miller, S. C.; Jarvi E. T.; Sabol, J. S.; McCarthy, J. R., Tettrahedron Lett. 1993, 34 (19), 3057.
- Brunaus, M.; Dell, C. P.; Owton, W. M., J. Fluorine Chem. 1994, 201.
- McClinton, M. A. ; Sik, V., J. Chem. Soc., Perkin Trans. I, 1992, 1891.
- Hodson, H. F.; Madge, D. J.; Slawin, A. N. Z.; Widdawson, D. A.; Williams, D. J., Tetrahedron, 1994, 50 (6), 1899.
- Stavber, S.; Zupan, M., J. Chem. Soc., Chem. Commun. 1994, 149.
- Stavber, S.; Sotler, J.; Zupan, M., Tettrahedron Lett. 1994, 35 (7), 1105.
Patents
- US 5459267 "1-substituted-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane salts and their application as fluorinating agents"
- US 55227493 "Fluorinated Sulfonamide Derivatives"
- US 5086178 "Fluorinated Diazabicycloalkane Derivatives"
- US 5473065 "Fluorinated Diazabicycloalkane Derivatives"
- US 5442084 "Method of Selective Fluorination"