Estrin (compound)
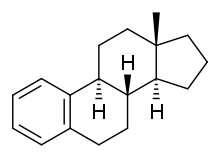
Estrin (American English), or oestrin (British English), also known as estra-1,3,5(10)-triene, is an estrane steroid. It is dehydrogenated estrane with double bonds specifically at the C1, C3, and C5(10) positions. Estrin is a parent structure of the estrogen steroid hormones estradiol, estrone, and estriol, which have also been known as dihydroxyestrin, ketohydroxyestrin, and trihydroxyestrin, respectively.[1][2]
 | |
| Names | |
|---|---|
| IUPAC name
(8S,9S,13S,14S)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene | |
| Other names
Estra-1,3,5(10)-triene; Estratriene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C18H24 | |
| Molar mass | 240.39 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Unlike its estrogen derivatives, estrin itself possesses minimal estrogenic activity, as hydroxyl and/or keto substitutions at the C3 and C17 positions are critical for high binding affinity to the estrogen receptors.[3][4] Estrin has been found to be on the order of 1,000-fold less potent than estradiol in inducing estrogenic responses in vitro.[5][6][3] In addition to estrin, estratrien-17β-ol, which lacks the 3-hydroxyl group of estradiol, and 3-hydroxyestratriene, which lacks the 17β-hydroxyl group of estradiol, both have measurable affinity for the estrogen receptor and are able to activate the receptor and induce progesterone receptor expression.[6][7][3]
The term estrin is also a synonym for estrogen.[8] It was coined by Sir Alan S. Parkes and C. W. Bellerby in 1926 to describe the hormone secreted from the ovaries that induces estrus in animals (i.e., estrogen).[8]
Structures of major endogenous estrogens
|
References
- Biskind, Morton S. (1935). "Commercial glandular products". Journal of the American Medical Association. 105 (9): 667. doi:10.1001/jama.1935.92760350007009a. ISSN 0002-9955.
- Fluhmann CF (1938). "Estrogenic Hormones: Their Clinical Usage". Cal West Med. 49 (5): 362–6. PMC 1659459. PMID 18744783.
- Anstead GM, Carlson KE, Katzenellenbogen JA (1997). "The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site". Steroids. 62 (3): 268–303. doi:10.1016/s0039-128x(96)00242-5. PMID 9071738.
- Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1395–. ISBN 978-1-60913-345-0.
- Jordan VC, Koch R (April 1989). "Regulation of prolactin synthesis in vitro by estrogenic and antiestrogenic derivatives of estradiol and estrone". Endocrinology. 124 (4): 1717–26. doi:10.1210/endo-124-4-1717. PMID 2924721.
- Brooks SC, Wappler NL, Corombos JD, Doherty LM, Horwitz JP (1987). "Estrogen structure-receptor function relationships". In Moudgil VK (ed.). Recent Advances in Steroid Hormone Action. Walter de Gruyter. pp. 443–466.
- Schwartz JA, Skafar DF (September 1993). "Ligand-mediated modulation of estrogen receptor conformation by estradiol analogs". Biochemistry. 32 (38): 10109–15. doi:10.1021/bi00089a029. PMID 8399136.
- Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 750–. ISBN 978-1-4511-4847-3.
